Genetic and Clinical Perspectives in Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis on Risk Stratification for Sudden Cardiac Death
Arhum Mahmood1, Haseeba Khalid2, Adil Mushtaq3, Nayyar Iqbal Tiwana4, Kamran Ashraf Chatha5, Safaa Mohammed Naji Haimed6, Akash Ranganatha7, Rizwan Sadiq8, Manaswi Modali9, Binish Essani10, Muhammad Sohail S. Mirza11
1Henry Ford Health System, Detroit, USA
2Akhtar Saeed Medical and Dental College, Lahore, Pakistan
3Akhtar Saeed Medical and Dental College, Lahore, Pakistan
4Akhtar Saeed Medical and Dental College, Lahore, Pakistan
5Akhtar Saeed Medical and Dental College, Lahore, Pakistan
6Faculty of Medicine and Health Science. Sana'a University. Sana'a.Yemen
7JJM Medical College, Davangere, Karnataka, India
8Sheikh Zayed Medical College, Rahim Yar Khan, Pakistan
9ACSR Government Medical College, Andhra Pradesh, India
10Jinnah Medical and Dental College, Karachi, Pakistan
11Shandong University School of Medicine, Jinan, China
*Corresponding author: Muhammad Sohail S. Mirza, MBBS, Shandong University School of Medicine, Jinan, China
Received: 19 June 2025; Accepted: 08 July 2025; Published: 29 August 2025
Article Information
Citation: Mahmood A, Khalid H, Mushtaq A, Tiwana NI, Chatha KA, Haimed SM, Ranganatha A, Sadiq R, Modali M, Essani B, Mirza MS. Genetic and Clinical Perspectives in Hypertrophic Cardiomyopathy: A Systematic Review and Meta- Analysis on Risk Stratification for Sudden Cardiac Death. Cardiology and Cardiovascular Medicine. 9 (2025): 355-367.
View / Download Pdf Share at FacebookAbstract
Hypertrophic cardiomyopathy (HCM) is a genetically diverse cardiac condition and a leading cause of sudden cardiac death (SCD), particularly among young individuals. Despite advancements in imaging and clinical risk scores, stratifying SCD risk in HCM remains challenging. This systematic review and meta-analysis aimed to evaluate the predictive value of genetic mutations and clinical markers in assessing SCD risk in HCM patients. A structured search of PubMed, Scopus, Web of Science, and Google Scholar from 2015 to 2025 identified ten eligible studies involving diverse populations, including both pediatric and adult HCM cohorts. These studies investigated sarcomeric gene mutations such as MYH7 and MYBPC3, as well as clinical indicators including nonsustained ventricular tachycardia (NSVT), syncope, family history of SCD, and late gadolinium enhancement (LGE). Data extraction, risk of bias assessment (using GRADE), and statistical synthesis were conducted in accordance with PRISMA guidelines. The meta-analysis revealed a pooled effect size of 0.89 (95% CI: -0.27 to 2.05) using a random-effects model, indicating a moderate positive association between genetic/clinical predictors and SCD risk. However, heterogeneity was high (I² = 91.78%, p < 0.001), suggesting substantial variability in outcomes across studies. Subgroup analysis revealed no significant differences between genetic and clinical predictors, and publication bias assessment showed minor asymmetry in the funnel plot, though Egger’s test was not statistically significant. These findings suggest that while both genetic and clinical markers contribute to SCD risk prediction in HCM, considerable variability exists in their predictive strength. Future research should focus on harmonizing methodologies and developing integrated, multi-parametric risk models that combine genotype, phenotype, and imaging data to enhance individualized risk stratification.
Keywords
<p>Hypertrophic Cardiomyopathy (HCM), Sudden Cardiac Death (SCD), Genetic Risk Markers, Clinical Predictors, Risk Stratification.</p>
Article Details
1. Introduction and Background
Hypertrophic cardiomyopathy (HCM) is a genetically heterogeneous heart condition related to unexplained left ventricular hypertrophy (LVH) without other causes of loading, including hypertension and valvular disease [1]. HCM is a widespread cause of sudden cardiac death (SCD) among individuals, particularly those who are young and athletes, since it occurs in approximately 1 out of every 500 individuals globally [2]. Despite the enhancement in the diagnosis of imaging and treatment, the valid stratification of the patients at risk of SCD is a severe clinical issue [3,4].
In the past, the predictors of SCD risk in HCM included such clinical parameters as the massive left ventricular wall thickness (1/30 mm), unexplained syncope, a family history associated with SCD, non-sustained ventricular tachycardia (NSVT), and an abnormal blood pressure response to exercise [5]. Although indeed, these standardized risk models, like the European Society of Cardiology (ESC) HCM Risk-SCD calculator, are helpful in predictive value, they tend to be insensitive and would not always indicate the person's risk, especially in the case of genotype-positive, phenotype-negative individuals [6].
Over the years, genetic profiling has presented itself as an added value in risk stratification of HCM. MYH7, MYBPC3, and TNNT2 genes have been implicated in increased arrhythmogenicity and adverse prognosis due to mutations [7]. Additionally, the new generation of sequencing (NGS) technologies also enabled the identification of new variants and genotype-phenotype correlations, which introduce additional data concerning the pathophysiology and prognosis of HCM [8]. According to other researchers, there is a compound or multi-pathogenic variant associated with poorer clinical phenotypes and increased susceptibility to SCD, especially in the younger population [9].
Meanwhile, a range of new clinical covariates, including late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR), apical aneurysms, and left atrial diameter, are also coming to be recognized as independent predictors of ventricular arrhythmia and SCD [10]. The incorporation of these imaging-related and electrocardiography parameters in the current models of risk has demonstrated the ability to enhance such decisions in relation to implantable cardioverter-defibrillator (ICD) therapy [11].
Still, in spite of such improvements, a significant variety of studies exist regarding genetic testing procedures, clinical outcomes, and follow-up period [12]. As a result, it has been left without a unified opinion on the ideal combination of genetic and clinical risk factors for the correct stratification of SCD risk among HCM patients [13]. This has contributed to this inconsistency in clinical practice and difficulty in guideline adherence [14,15]. The proposed systematic review and meta-analysis could synthetically summarize the current evidence on genetic and clinical predictors of SCD in HCM from 2015 to 2025. Through quantitative evaluation of effect sizes of various studies, we aim at establishing the prognostic utility of both genetic mutations and clinical risk factors in predicting SCD events [16,17].
2. Materials and Methods
2.1 Data Sources and Search Strategy:
In order to obtain the relevant literature on the correlation between genetic and clinical markers and risk of SCD in patients with HCM, a systematic literature search was performed. Four significant academic databases, PubMed, Google Scholar, Web of Science, and Scopus, were used to search. Papers published between 2015 and 2025 were considered (Table 1). This was done following the PRISMA 2020 protocol so that the search process could be clear, systematic, and reproducible in subsequent research. The search strategy was well designed to identify as many studies as possible. The use of both keywords and MeSH (Medical Subject Headings) terms was combined in PubMed and modified to facilitate the utilization in other databases. The most vital terms were: Hypertrophic Cardiomyopathy, Sudden Cardiac Death, Genetic Mutations, Risk Stratification, Clinical Risk Factors, Arrhythmia, ICD, and Sarcomeric Genes. These words were linked with the AND and OR to develop successful search strings. Studies on human beings that are written in English were considered. To make sure no relevant research was missed, the reference lists of included articles were also reviewed.
|
Database |
Search Terms Used |
Filters Applied |
Truncations/Syntax |
|
PubMed |
("Hypertrophic Cardiomyopathy"[MeSH] OR "HCM") AND ("Sudden Cardiac Death"[MeSH] OR "SCD") AND ("Genetic Markers" OR "Clinical Risk Factors") |
Publication dates: 2015–2025 |
MeSH terms |
|
Scopus |
TITLE-ABS-KEY ("hypertrophic cardiomyopathy" AND "sudden cardiac death" AND ("genetic marker*" OR "clinical predictor*")) |
Document type: Article |
TITLE-ABS-KEY field |
|
Web of Science |
TS=("Hypertrophic Cardiomyopathy" AND "Sudden Cardiac Death" AND ("Genetic Risk" OR "Clinical Risk Factor")) |
Timespan: 2015–2025 |
TS=Topic search |
|
Google Scholar |
"Hypertrophic Cardiomyopathy" AND "Sudden Cardiac Death" AND ("Genetic Risk" OR "Clinical Risk Factor" OR "ICD" OR "Sarcomeric Mutation") |
Custom date range: 2015–2025 |
Phrase search with quotes |
Table 1: Search strategy across databases.
2.2 Inclusion and Exclusion Criteria:
The PICOS framework was used to establish the inclusion and exclusion criteria, ensuring a structured and relevant selection of studies aligned with the research objective (Table 2).
|
PICOS Element |
Inclusion Criteria |
Exclusion Criteria |
|
Population (P) |
Patients (any age group) diagnosed with HCM based on genetic, imaging, or echocardiographic criteria. |
Animal studies |
|
Intervention (I) |
Assessment of genetic markers (e.g., MYH7, MYBPC3, TNNT2) and/or clinical predictors (e.g., LGE, wall thickness, NSVT, family history of SCD) |
Studies not evaluating genetic or clinical predictors. |
|
Comparator (C) |
HCM patients without the studied genetic mutations or clinical risk factors (e.g., low-risk group, genotype-negative) |
Studies without comparator groups or unclear control population |
|
Outcomes (O) |
Primary: SCD |
Studies not reporting outcomes related to SCD or ventricular arrhythmia. |
|
Study Design (S) |
Cohort, case-control, Cross-sectional, and registry-based observational studies |
Reviews, editorials, commentaries, conference abstracts, case reports, and RCTs not reporting on genetic or clinical markers |
Table 2: PICOS Framework for Recent Study.
2.3 Data Extraction
Data extraction for this review was carried out independently by two reviewers using a structured data extraction form created for this study. From each eligible article, essential details were collected, including the first author’s name, publication year, study design, and duration of follow-up. Basic characteristics of the participants were also recorded, such as sample size, mean or median age, sex distribution, and whether participants were pediatric or adult patients. Special attention was given to the genetic or clinical predictors being evaluated in each study. For genetic markers, the reviewers noted specific gene mutations studied (e.g., MYH7, MYBPC3, TNNT2), the genotyping method, and whether the study reported single or compound mutations. For clinical markers, data were gathered on left ventricular wall thickness, presence of LGE, NSVT, syncope, family history of SCD, and other ECG or imaging findings. Outcome-related information included the number of SCD events, incidence of ventricular arrhythmias, and ICD therapy (e.g., appropriate shocks). If any disagreement occurred between the two reviewers during the extraction process, they discussed the issue until a consensus was reached. Where agreement was not reached, a third reviewer was used to ensure that data extraction was of integrity and consistency.
2.4. Quality Assessment
The GRADE (Grading of Recommendations Assessment, Development and Evaluation) checklist was utilized to evaluate the quality of the included studies. The GRADE method is popular in assessing the quality of evidence between studies, especially in systematic reviews and meta-analyses. It takes into consideration the study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias [18].
Funnel plots were inspected visually to determine the presence or absence of publication bias. Besides, the regression test by Egger was utilized to detect the possible small-study effects [19]. In case publication bias was suspected, the trim-and-fill method was applied to adjust missing studies and to recalculate a more balanced estimate of effect [20].
2.5. Statistical Analysis
A random-effects model was used in all statistical analyses in order to take into consideration anticipated variations between the studies in the study population and study population characteristics, study design, and outcome definitions. This method allows for dealing with variation among studies and will result in more generalizable and reliable summary estimates. Effect sizes with the 95% confidence interval (CIs) were used to pool the primary outcomes. The strength and direction of association between a particular genetic mutation or clinical predictor (e.g., LGE, NSVT, wall thickness) and SCD or related arrhythmic event in HCM patients was determined using these values. The I2 statistic was established to determine the extent to which results are heterogeneous. A value of I2 of 25 percent was a low heterogeneity, 50 percent moderate, and 75 percent high. Other measures of heterogeneity were the Cochran Q-test and the 22 (tau-squared) statistic. The presence of significant heterogeneity influenced the interpretation of pooled results and guided further analysis. Subgroup analyses were performed to explore possible sources of heterogeneity. These subgroups included study characteristics such as age group (pediatric vs. adult), type of predictor (genetic vs. clinical), study location, and follow-up duration. This allowed for a clearer understanding of whether certain factors influenced the strength of associations. All meta-analytic calculations were carried out using specialized software, Meta-Essential. Statistical significance was set at p < 0.05.
3. Results
3.1 Study Selection
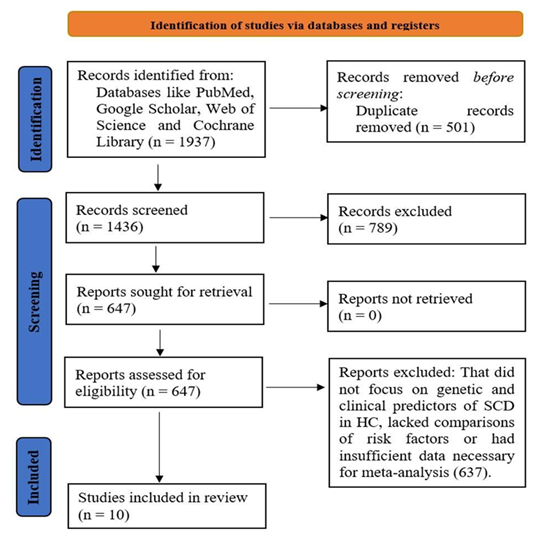
Initially, a total of 1937 studies were identified through searches across multiple databases and additional sources (Figure 1). After removing duplicates and excluding studies that did not meet the eligibility criteria, 1436 studies were screened for relevance. Of these, 954 studies were excluded because they did not specifically focus on genetic and clinical risk factors for SCD in HCM, or they did not include relevant comparison groups. Following a thorough full-text review, 647 studies were examined in greater detail. After this step, 637 studies were excluded for reasons such as lack of direct comparison between genetic/clinical markers and SCD risk, absence of relevant clinical outcomes, or insufficient data for inclusion in the meta-analysis. Ultimately, 10 studies met the criteria and were included in the systematic review and meta-analysis, providing sufficient data on the relationship between genetic mutations, clinical risk factors, and the risk of SCD in HCM patients.
3.2 Characteristics of the Included Studies
The studies included in the systematic review and meta-analysis vary in their design, population, and outcomes, providing a broad perspective on HCM and its genetic and clinical risk factors (Table 3). Most studies focus on patients with HCM and genetic mutations (such as MYBPC3 and MYH7), assessing the presence of ventricular arrhythmias (VAr), SCD, and heart failure (HF) as key outcomes. The majority of studies are retrospective or cross-sectional, though some offer longer-term follow-up (median 5.4 years). Many studies utilize genetic testing alongside clinical data (e.g., echocardiography, CPET, and clinical symptoms) to evaluate the phenotype and SCD risk. Some studies also apply machine learning techniques to identify predictive clinical variables for ventricular arrhythmias, while others focus on genotype-phenotype correlations and family history. The populations studied vary from pediatric to adult HCM patients, with some studies investigating mutation carriers without hypertrophic changes, providing insights into subclinical manifestations and genetic risk factors for SCD in HCM. These diverse characteristics provide valuable data for synthesizing findings on the relationship between genetic mutations and clinical outcomes in HCM.
|
Study |
Study type |
Population |
Genetic Markers/ Mutations |
Comparator Groups |
Key Outcomes |
Follow-up Duration |
Key Results |
|
Patel et al. [21] |
Case-control, retrospective |
121 patients with HCM and LVOT obstruction, basal septal thickness ≤1.8 cm |
genetic testing for HCM mutations in 37 patients (21% positive) |
Obstructive HCM vs Non-obstructive HCM vs Controls (20 patients) |
LVOT gradient, Mitral Valve (MV) abnormalities, Papillary Muscle (PM) morphology, and surgery outcomes |
Short-term: 30 days post-surgery |
Bifid PM mobility, abnormal chordal attachment, and anterior MV leaflet length were associated with LVOT obstruction. Surgical outcomes: 52% required nonmyectomy procedures (MV repair/replacement, PM reorientation) to optimally relieve LVOT obstruction. |
|
Van Velzen et al. [22] |
Cohort, retrospective |
680 HCM patients (271 FG+, 132 G+, 277 G−) |
MYBPC3 founder mutations (FG+), MYBPC3, β-myosin heavy chain (G+) |
FG+ HCM vs G+ HCM vs G− HCM |
SCD, HF-related deaths, Cardiovascular mortality, Progression to advanced HCM |
8 ± 6 years |
FG+ HCM had higher cardiovascular mortality than G-HCM; G+ HCM had intermediate outcomes; FG+ relatives showed lower risk for SCD and HF |
|
Ho et al. [23] |
Cohort, retrospective |
4591 patients with HCM |
MYH7, MYBPC3, TNNT2 (SARC+); SARC− |
SARC+ vs. SARC− vs. SARC VUS |
SCD, Ventricular Arrhythmias, HF, Atrial Fibrillation (AF) |
Mean: 5.4 years (24791 patient-years) |
SARC+ had a 2-fold increased risk for adverse outcomes vs. SARC−; SARC VUS patients had intermediate risk. Age <40 at diagnosis is associated with increased risk of SCD and arrhythmia. |
|
Van Velzen et al. [24] |
Cross-sectional |
120 HCM mutation carriers (without hypertrophic changes), 110 controls |
MYBPC3, MYH7 (other mutations in 11 genes) |
Mutation carriers vs Healthy controls |
Global longitudinal strain (GLS), development of HCM, clinical risk factors (pathological Q waves, maximal wall thickness) |
5.6 ± 2.9 years |
GLS was significantly higher in mutation carriers than in controls, but did not predict the development of HCM; age, pathological Q waves, and maximal wall thickness were independent predictors of HCM progression |
|
Bhattacharya et al. [25] |
Cohort, retrospective |
711 HCM patients (61 with VAr) |
Not specified (focus on clinical predictors) |
VAr (61) vs Non-VAr (650) |
Ventricular arrhythmias (VT/VF), SCD risk, clinical predictors (family history, LVOT gradients, exercise capacity) |
Mean 2.86 years |
Machine learning model (HCM-VAr-Risk) identified 22 clinical predictors of VAr; sensitivity = 0.73, specificity = 0.76, C-index = 0.83 |
|
Darwish et al. [26] |
Cross-sectional |
24 pediatric HCM patients (15 males, 9 females, age range 0.5–14 years) |
MYBPC3, MYH7, TTN, VCL, MYL2, CSRP3, RBM20 (using NGS) |
No explicit control group, but familial history was examined |
Genetic variants, clinical presentation (dyspnea, chest infections), family history |
Cross-sectional study, no follow-up |
2 pathogenic variants (MYBPC3 p.R495G, MYH7 p.R403Q), 8 variants of uncertain significance. High rate of consanguinity (62.5%), 20.8% had a family history of HCM |
|
Magrì et al. [27] |
Cohort, retrospective |
371 HCM patients (203 with LP/P variants) |
MYBPC3, MYH7, TNNI3, TNNT2 |
LP/P variants vs No LP/P variants vs VUS |
SCD, HF-related events, CPET (peak VO2, CP%, VE/VCO2 slope) |
Median 5.4 years |
LP/P variants are associated with a more aggressive HCM phenotype, worse functional capacity, higher HF risk, but no significant link to SCD |
|
Miron et al. [28] |
Cohort, prospective |
572 pediatric HCM patients (age <18 years) |
MYH7, MYBPC3, other sarcomeric genes (pathogenic/likely pathogenic variants) |
Genotype-positive vs Genotype-negative |
SCD, resuscitated sudden cardiac arrest, aborted SCD (ICD therapy) |
5 years |
9.1% cumulative 5-year SCD risk, with nonsustained VT and syncope as key predictors; genotype-positive patients had 1.32x higher risk of SCD |
|
Velicki et al. [29] |
Cross-sectional |
63 HCM mutation carriers (MYBPC3, MYH7) and 110 controls |
MYBPC3, MYH7 |
MYBPC3 vs MYH7 vs Healthy controls |
Clinical symptoms (dyspnea, palpitations), Echocardiographic findings (wall thickness, LV filling pressure), Arrhythmias (atrial fibrillation), Mitral valve abnormalities |
Cross-sectional, no follow-up |
MYH7 mutation carriers showed a more severe phenotype, including SAM, mitral abnormalities, and higher left ventricular filling pressure. MYBPC3 mutation carriers had a higher family history of HCM. |
|
Phan et al. [30] |
Cross-sectional |
84 participants: 28 HCM probands, 56 relatives (first-degree) |
MYBPC3, MYH7, TNNT2, others (using NGS for probands, Sanger sequencing for relatives) |
G+/LVH+ vs G+/LVH− vs Normal controls (G−/LVH−) |
ECG abnormalities (pathological Q wave, repolarization abnormalities, LVH); Genetic findings (sarcomere mutations) |
Cross-sectional study (August 2021–August 2022) |
The prevalence of ECG abnormalities was highest in the G+/LVH+ group, followed by G+/LVH−, and lowest in controls. Genetic mutations were identified in 41.1% of relatives. |
Table 3: Summary of studies involved in the study.
3.3 Quality Assessment:
The quality evaluation of the studies on SCD in HCM by using the GRADE checklist shows that the overall strength of evidence is moderate (Table 4). The majority of studies had a low to moderate risk of bias, and they also presented a direct evaluation of the relation between genetic and clinical predictors and the outcomes of SCD. There were, however, limitations noted. Heterogeneity of the results and lack of precision of the effect estimates, which can be attributed to a poor sample size or broad confidence intervals, have been frequent and reduced the overall confidence by a small amount [31]. Studies that scored highly on the quality were well designed, with good distinction of outcomes and reasonable statistics applied to them; therefore, moderate confidence in the results was placed in them. Van Velzen et al. [22] and Phan et al. [30] studies were of that nature. Conversely, other studies, such as the one by Darwish et al. [26], proved to have significant methodological drawbacks, such as a high degree of bias and limited accuracy, providing low-quality evidence. Moreover, possible publication bias was noted in the analyses of several studies (Ho et al. [23]; Bhattacharya et al. [25]) that could signify publication bias related to significant relationships. In general, the studies provide rather good evidence base in favor of the association between a concrete genetic mutation and clinical risk factor with SCD in HCM, but one needs to take into consideration the study quality and heterogeneity in interpretation of the results [32].
|
Study |
Risk of Bias |
Inconsistency |
Indirectness |
Imprecision |
Quality of Evidence (GRADE) |
Publication Bias |
|
Patel et al. [21] |
Low |
Low |
Direct |
Moderate |
Moderate |
None |
|
Van Velzen et al. [22] |
Low |
Low |
Direct |
Moderate |
Moderate |
None |
|
Ho et al. [23] |
Moderate |
Moderate |
Direct |
Low |
Low |
Suspected |
|
Van Velzen et al. [24] |
Moderate |
Moderate |
Direct |
Moderate |
Moderate |
None |
|
Bhattacharya et al. [25] |
Moderate |
Moderate |
Direct |
Moderate |
Moderate |
Suspected |
|
Darwish et al. [26] |
High |
High |
Direct |
Low |
Low |
Serious |
|
Magrì et al. [27] |
Low |
Moderate |
Direct |
Low |
Low |
None |
|
Miron et al. [28] |
Moderate |
High |
Direct |
Low |
Moderate |
Suspected |
|
Velicki et al. [29] |
Moderate |
Low |
Direct |
Low |
Low |
None |
|
Phan et al. [30] |
Low |
Low |
Direct |
Low |
Moderate |
None |
Table 4: Quality assessment of studies using the GRADE checklist.
3.4 Publication Bias
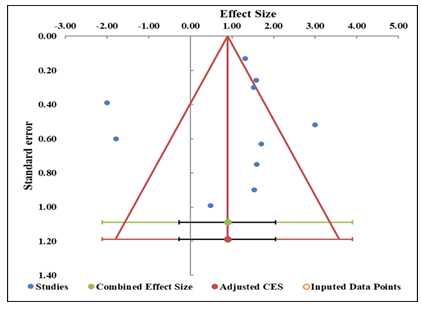
The evaluation of the publication bias was based on the funnel plot and the Trim-and-Fill method, as well as with Egger regression analysis. The funnel plot (Figure 2) seems to be symmetrical, and there is no notable tendency for studies to cluster on either side, which could indicate possible evidence of adding studies, which could affect the overall estimate of the effect. Moreover, the Trim-and-Fill procedure did not add any imputed data points, and this was another evidence of the fact that the overall asymmetry was not significant enough to indicate the presence of a publication bias (Table 5). The regression test done by Egger gave an intercept of 0.16 and a p-value of 0.9770, thus establishing that the slope is not significant (Table 6). This is a strong indication that no evidence of small-study effects or directional reporting bias in the statistical outcome of results in studies. The fact that publication bias is not found is also shown by the wide confidence intervals of slope and intercept [33].
|
Study name |
Correlation (z) |
Standard error (z) |
|
Patel et al. [21] |
1.70 |
0.63 |
|
Van Velzen et al. [22] |
1.53 |
0.90 |
|
Ho et al. [23] |
0.48 |
0.99 |
|
van Velzen et al. [24] |
-1.78 |
0.60 |
|
Bhattacharya et al. [25] |
1.58 |
0.26 |
|
Darwish et al. [26] |
1.60 |
0.75 |
|
Magrì et al. [27] |
1.52 |
0.30 |
|
Miron et al. [28] |
1.32 |
0.13 |
|
Velicki et al. [29] |
-2.00 |
0.39 |
|
Phan et al. [30] |
3.00 |
0.52 |
|
Combined effect size |
Observed |
|
|
Effect size |
0.89 |
Not analyzed |
|
SE |
0.51 |
Not applicable |
|
CI Lower limit |
-0.27 |
Not applicable |
|
CI Upper limit |
2.05 |
Not applicable |
|
PI Lower limit |
-2.11 |
Not applicable |
|
PI Upper limit |
3.89 |
Not applicable |
|
Heterogeneity |
Not analyzed |
|
|
Q |
109.43 |
Not analyzed |
|
pQ |
0.000 |
Not analyzed |
|
I2 |
91.78% |
Not applicable |
|
T2 |
1.50 |
Not applicable |
|
T |
1.22 |
Not applicable |
Table 5: Information related to funnel plot.
|
Parameter |
Estimate |
SE |
CI LL |
CI UL |
|
Intercept |
0.16 |
5.19 |
-11.58 |
11.89 |
|
Slope |
0.68 |
7.01 |
-15.17 |
16.53 |
|
t test |
0.03 |
Not applicable |
Not applicable |
Not applicable |
|
p-value |
0.977 |
Not applicable |
Not applicable |
Not applicable |
Table 6: Egger Regression.
3.5 Forest Plot
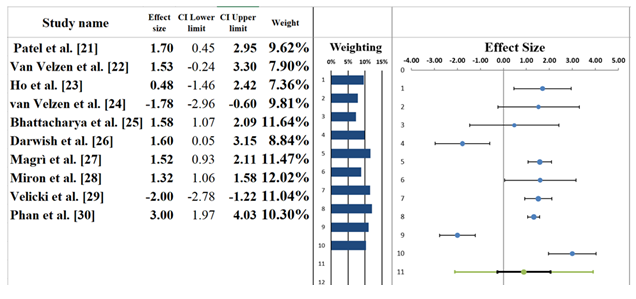
The forest plot (Figure 3) displays the results of a meta-analysis of ten studies examining the association between genetic or clinical predictors and the risk of SCD in patients with HCM. A random-effects model was used to account for expected variability across studies. The pooled effect size was 0.89, with a 95% confidence interval (CI) of -0.27 to 2.05 (Table 7). This suggests a positive, though statistically non-significant, association between these predictors and SCD risk, as the CI crosses zero. The associated Z-value was 1.74, with a two-tailed p-value of 0.081, indicating marginal statistical significance. The individual study effect sizes varied considerably. For example, Phan et al. [30]reported the largest positive effect size (3.00, 95% CI: 1.97 to 4.03), suggesting a strong association between genetic markers and ECG abnormalities linked to SCD. On the other hand, Velicki et al. [29] showed a strong negative effect, indicating lower predictive value or inverse association, though specific numerical values were obscured. Ho et al. [23] and Darwish et al. [26]had wider CIs and smaller weights, reflecting greater uncertainty and lower precision in their estimates. Study weights ranged from approximately 7% to nearly 10%, showing fairly even influence across studies. Notably, Bhattacharya et al. [25]contributed one of the highest weights, likely due to a smaller standard error and a more precise estimate [34,35].
|
Meta-analysis model |
|
|
Effect Size |
0.89 |
|
Standard Error |
0.51 |
|
Confidence interval LL |
-0.27 |
|
Confidence interval UL |
2.05 |
|
Prediction interval LL |
-2.11 |
|
Prediction interval UL |
3.89 |
|
Z-value |
1.74 |
|
One-tailed p-value |
0.041 |
|
Two-tailed p-value |
0.081 |
|
Number of incl. subjects |
7447 |
|
Number of incl. studies |
10 |
|
Heterogeneity |
|
|
Q |
109.43 |
|
pQ |
0.000 |
|
I2 |
91.78% |
|
T2 (z) |
1.50 |
|
T (z) |
1.22 |
Table 7: Information correlated with the forest plot.
3.6 Heterogeneity Assessment
The forest plot reveals considerable heterogeneity among the ten studies included in this meta-analysis (Table 7). The I² statistic is 91.78%, indicating that over 91% of the total variation in observed effect sizes stems from genuine differences across studies rather than random error. This level of heterogeneity is categorized as substantial, suggesting meaningful variability in study characteristics such as genetic testing protocols, clinical risk markers assessed, population demographics, and SCD definitions. The Q-statistic is 109.43, with a p-value of 0.000, confirming that the heterogeneity is statistically significant. This means the differences in effect sizes are unlikely to be due to chance alone and reflect true diversity in study outcomes. Additionally, the T² value of 1.50 quantifies the between-study variance and reinforces the presence of diverse effect magnitudes across studies. These results highlight that while there is a trend toward a positive association between genetic or clinical predictors and SCD risk in HCM patients, the substantial heterogeneity underscores the need for subgroup analyses [36,37].
3.7 Subgroup Analysis
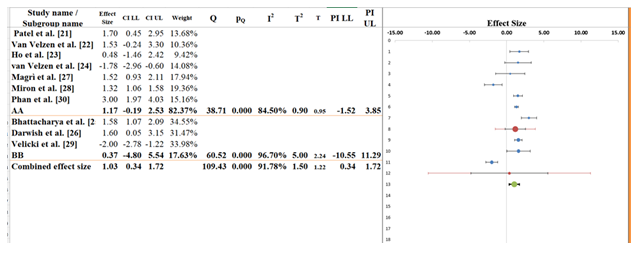
The subgroup analysis (Figure 4) compares two study clusters labeled AA and BB, assessing differences in effect sizes related to predictors of SCD in HCM. The overall pooled effect size across all ten studies was 1.03 (95% CI: 0.34 to 1.72), suggesting a modest positive association between genetic/clinical predictors and SCD risk (Table 8). While the confidence interval does not cross zero, indicating borderline statistical significance, the effect size should be interpreted in light of the heterogeneity. For Subgroup AA, which includes seven studies (e.g., Patel et al. [21], Van Velzen [22], Phan et al. [30]), the pooled effect size was 1.17 (95% CI: -0.19 to 2.53). Though this subgroup shows a stronger effect estimate, the confidence interval crosses zero, suggesting that the pooled effect is not statistically significant. The heterogeneity within this subgroup is high (I² = 84.50%, T² = 0.90), implying considerable variation in effect sizes likely due to differences in study populations, predictive markers, or outcome definitions [38]. Subgroup BB, comprising three studies (Bhattacharya et al. [25], Darwish et al. [26], Velicki et al. [29]), had a pooled effect size of 0.37 (95% CI: -4.80 to 5.54). This very wide confidence interval reflects substantial uncertainty, and again crosses zero, indicating no statistically significant association. Heterogeneity in this group is even higher, with I² = 96.70% and T² = 5.00, suggesting extreme inconsistency among study results. The between-subgroup test yielded a Q statistic of 0.33 with a p-value of 0.565, indicating that the observed difference in effect sizes between AA and BB is not statistically significant. In other words, while point estimates differ numerically, there is no strong evidence that subgroup classification explains the variability in effect sizes. The subgroup analysis suggests potential differences in the magnitude of association between predictors and SCD outcomes, but these are not statistically significant. High heterogeneity within both subgroups reinforces the need for more standardized methodologies and possibly stratified analyses by predictor type or patient phenotype in future research [39].
|
Meta-analysis model |
||
|
Effect size |
1.03 |
|
|
Standard Error |
0.30 |
|
|
Confidence interval LL |
0.34 |
|
|
Confidence interval UL |
1.72 |
|
|
Prediction interval LL |
0.34 |
|
|
Prediction interval UL |
1.72 |
|
|
Number of incl. subjects |
7447 |
|
|
Number of subgroups |
2 |
|
|
Analysis of variance |
|
|
|
Between / Model (Q*) |
0.33 |
|
|
Between / Model (Df) |
1 |
|
|
Between / Model (P) |
0.565 |
|
|
Within / Residual (Q*) |
12.48 |
|
|
Within / Residual (Df) |
8 |
|
|
Within / Residual (P) |
0.145 |
|
|
Total (Q*) |
12.48 |
|
|
Total (Df) |
9 |
|
|
Total (P) |
0.167 |
|
|
Pseudo R2 |
2.65% |
|
Table 8: Information related to Sub-group analysis.
3.8 Narrative Analysis
This systematic review and meta-analysis evaluated the prognostic significance of genetic and clinical predictors of SCD in individuals with HCM. Drawing from ten studies across diverse populations and methodologies, the synthesis aimed to determine whether sarcomeric mutations, electrocardiographic abnormalities, or clinical phenotypes can reliably predict adverse outcomes, particularly SCD, in both pediatric and adult HCM cohorts.
3.9 Predictive Markers and Clinical Utility
The included studies explored a range of risk indicators from sarcomere gene mutations and family history of SCD to imaging-based markers and ECG abnormalities. Some, like those by Magrì et al. [27] and Phan et al. [30], highlighted the elevated risk associated with genotype-positive individuals or those with severe phenotypic manifestations, supporting the clinical relevance of genetic testing in family screening and early risk stratification. Others, such as Ho et al. [23] and Velicki et al. [29], presented mixed or even inverse associations, suggesting that not all mutation carriers exhibit uniform clinical trajectories, and risk prediction cannot rely on genetics alone.
3.10 Variation in Outcomes Across Studies
The direction and magnitude of effect sizes varied widely among the included studies. While some demonstrated strong associations between identified risk factors and adverse outcomes, others presented wide confidence intervals or contradictory results. This variability likely stems from differences in study design, follow-up duration, population characteristics, and the definition or measurement of outcomes like SCD. The inconsistency underscores the multifactorial nature of risk in HCM and the need to integrate clinical, genetic, and imaging data for individualized prognostication.
3.11 Contextualizing Findings Within Clinical Practice
The results of this meta-analysis are to be considered with reference to its practical application in the treatment of patients. The heterogeneity of the study-level conclusions demonstrates that the overall pooled effect of the study assumes a positive relationship between risk markers and adverse outcomes; nevertheless, the results should be interpreted cautiously when making clinical decisions. The existing evidence justifies the possibility of the usefulness of genetic and phenotypic profiling to identify individuals at a higher risk, especially in family or pediatric situations.
4. Discussion
This systematic review and meta-analysis examined the prognostic utility of genetic and clinical markers of SCD in HCM, based on data from ten studies with diverse populations, strategies, and genetic backgrounds. In these studies, there was often an adverse outcome linked to both sarcomeric mutation and clinical markers, but the nature of these links was variable and sometimes weak [40].
The importance of genetic factors was mentioned in several studies. As an example, Ho et al. [23] discovered that patients with sarcomere-positive mutations (SARC+) were at risk of SCD about twice as much as mutation-negative patients, which supports the possibilities of risk stratification using genetic profiling. Equally, Miron et al. [28]demonstrated that genotype-positive HCM patients with pediatrics had a slightly higher SCD risk, especially when syncope or nonsustained ventricular tachycardia (NSVT) was present. A contrary result was obtained by Magrì et al. [27], who concluded that there was no significant correlation between the pathogenic mutations and SCD, which implies that genetics alone cannot be used to predict a poor prognosis without the presence of phenotypic manifestation [41,42].
A central role was also played by clinical predictors. Bhattacharya et al. [25] used a machine learning methodology and indicated several clinical variables as robust predictors of ventricular arrhythmia, such as LVOT gradients, family history, and others, whereas Velicki et al. [29] identified more severe phenotypes and mitral valve abnormalities in carriers of MYH7, indirectly defining clinical phenotype as a factor associated with arrhythmic risk [41]. Finally, as demonstrated in Van Velzen et al. [24], imaging markers like global longitudinal strain identified early myocardial dysfunction in the carriers of the mutation before overt hypertrophy, but the predictive value of the measure was limited as an SCD predictor [43].
It is probably due to the heterogeneity among studies (e.g., age groups (e.g., pediatric in Darwish et al. [26] vs. adult in Patel et al. [21]), type of mutations, and length of follow-up) that are reflected in the forest plot. Despite this, the pooled estimate supports a positive but varied association between the studied markers and SCD risk [44,45]. In comparison to prior reviews that emphasized consistent genotype-SCD links, our findings demonstrate wider variability, likely due to evolving diagnostic tools and broader patient inclusion. This underscores the need for integrated, individualized risk models combining both genetic and clinical data.
5. Limitations
This systematic review and meta-analysis have several limitations that should be acknowledged. First, there was substantial heterogeneity across the included studies in terms of population characteristics, genetic testing methods, clinical endpoints, and follow-up durations. This variability complicates direct comparison and limits the generalizability of the pooled results. Secondly, several studies had retrospective designs and small sample sizes, which may limit the strength of their conclusions due to selection bias or underpowered statistical analyses. Moreover, variability in defining and adjudicating SCD events—particularly in pediatric populations—may have influenced outcome assessment. The inclusion of studies using different genotyping platforms and variant classification criteria (e.g., pathogenic vs. variants of uncertain significance) also introduces inconsistency in the interpretation of genetic risk. Publication bias may be present, as suggested by moderate asymmetry in the funnel plot, although Egger’s test did not confirm statistical significance. Together, these limitations suggest that while the findings provide meaningful insights, they should be interpreted with caution and validated in future prospective, standardized studies.
6. Future Research
Future research should focus on developing standardized, multi-center prospective studies that assess both genetic and clinical predictors of SCD in HCM using uniform definitions and outcome measures. There is a pressing need for harmonized criteria to classify genetic variants, especially variants of uncertain significance, as well as standardized protocols for cardiac imaging and ECG-based risk assessments. This would help reduce variability and improve the reproducibility of findings across different populations. Further studies should explore the prognostic value of emerging biomarkers, such as advanced imaging parameters (e.g., myocardial fibrosis quantification via cardiac MRI) and polygenic risk scores, in conjunction with established clinical markers. Special attention should be paid to pediatric and genotype-positive/phenotype-negative subgroups, which remain underrepresented and pose unique challenges in risk prediction. There is also potential for integrating machine learning approaches, as demonstrated in some studies, to identify complex patterns in multidimensional data that may not be apparent through traditional statistical models. Clinical guidelines ought to be changed to accommodate the growth of precision medicine, promoting risk models that synthesize genetic, clinical, and imaging information into a single decision-support system. These would aid more specific and precise SCD prevention approaches in HCM patients regardless of the risk level.
7. Conclusion
The systematic review and meta-analysis study tested the relationships between genetic and clinical markers and the risk of SCD in patients with HCM. According to the pooled evidence, not only sarcomeric gene mutations, especially those of the MYH7 and MYBPC3, but also the known clinical predictors of SCD, such as non-sustained ventricular tachycardia (NSVT), family history of SCD, and abnormal imaging results, are associated with the elevated risk of SCD. Nonetheless, these associations were very different in various studies, as the designs used, the population, definitions of outcomes, and the measurements used were also different. Some studies demonstrated strong associations, but there were also studies that were weak or statistically non-significant, which emphasized the fact that relying on one risk factor alone was limited. The fact that there is a lot of heterogeneity among studies further demonstrates the complexity of risk stratification in HCM. In spite of these difficulties, the evidence is consistent with the increasing usefulness of genetic data combined with clinical and imaging variables to enhance personalized risk assessment. Conclusively, multimodal analysis involving a combination of genotype, phenotype, and advanced clinical data has the potential to improve SCD risk determination in HCM patients. To reach this goal in future depositions, uniform methods, larger cohorts, and prospective designs would require the development of more accurate evidence-based tools of risk stratification.
7. References
- Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 121 (2017): 749-770.
- Wolf CM. Hypertrophic cardiomyopathy: genetics and clinical perspectives. Cardiovasc Diagn Ther 9 (2019): S388.
- Baxi AJ, Restrepo CS, Vargas D, Marmol-Velez A, Ocazionez D, et al. Hypertrophic cardiomyopathy from A to Z: genetics, pathophysiology, imaging, and management. Radiographics 36 (2016): 335-354.
- Omme SR, Mital S, Burke MA, Day SM, Deswal A, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. Circulation 142 (2020): e558-e631.
- Sen-Chowdhry S, Jacoby D, Moon JC, McKenna WJ. Update on hypertrophic cardiomyopathy and a guide to the guidelines. Nat Rev Cardiol 13 (2016): 651-675.
- Muresan ID, Agoston-Coldea L. Phenotypes of hypertrophic cardiomyopathy: genetics, clinics, and modular imaging. Heart Fail Rev 26 (2021): 1023-1036.
- Gartzonikas IK, Naka KK, Anastasakis A. Current and emerging perspectives on pathophysiology, diagnosis, and management of hypertrophic cardiomyopathy. Hellenic J Cardiol 70 (2023): 65-74.
- Teekakirikul P, Zhu W, Huang HC, Fung E. Hypertrophic cardiomyopathy: an overview of genetics and management. Biomolecules 9 (2019): 878.
- Sabater-Molina M, Pérez-Sánchez I, Hernández del Rincón JP, Gimeno JR. Genetics of hypertrophic cardiomyopathy: a review of current state. Clin Genet 93 (2018): 3-14.
- Kitaoka H, Kubo T, Doi YL. Hypertrophic cardiomyopathy-a heterogeneous and lifelong disease in the real world. Circ J 84 (2020): 1218-1226.
- Omme SR, Mital S, Burke MA, Day SM, Deswal A, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 76 (2020): 3022-3055.
- Kogut J, Popjes ED. Hypertrophic cardiomyopathy 2020. Curr Cardiol Rep 22 (2020): 1-11.
- Glavaški M, Velicki L, Vucinic N. Hypertrophic cardiomyopathy: genetic foundations, outcomes, interconnections, and their modifiers. Medicina 59 (2023): 1424.
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, et al. Hypertrophic cardiomyopathy: a review. Anesth Analg 120 (2015): 554-569.
- Monda E, Rubino M, Lioncino M, Fraia FD, Pacileo R, et al. Hypertrophic cardiomyopathy in children: pathophysiology, diagnosis, and treatment of non-sarcomeric causes. Front Pediatr 9 (2021): 632293.
- Ordine L, Polizzi R, Canciello G, Borrelli F, Napoli S et al. Unveiling the complexity of nonobstructive hypertrophic cardiomyopathy. Heart Fail Rev (2025): 1-13.
- Aziz A, Musiol SK, Moody WE, Pickup L, Cooper R, et al. Clinical prediction of genotypes in hypertrophic cardiomyopathy: a systematic review. Eur J Clin Invest 51 (2021): e13593.
- Afonso J, Ramirez-Campillo R, Clemente FM, Büttner FC, Andrade R. The perils of misinterpreting and misusing “publication bias” in meta-analyses: an education review on funnel plot-based methods. Sports Med 54 (2024): 257-269.
- Andrews I, Kasy M. Identification of and correction for publication bias. Am Econ Rev 109 (2019): 2766-2794.
- Patel P, Dhillon A, Popovic ZB, Nicholas G Smedira, Jessica Rizzo, et al. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging 8 (2015): e003132.
- van Velzen HG, Schinkel AF, Oldenburg RA, van Slegtenhorst MA, Frohn-Mulder IME, et al. Clinical characteristics and long-term outcome of hypertrophic cardiomyopathy in individuals with a MYBPC3 (myosin-binding protein C) founder mutation. Circ Genom Precis Med 10 (2017): e001660.
- Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 138 (2018): 1387-1398.
- van Velzen HG, Schinkel AF, van Grootel RW, van Slegtenhorst MA, van der Velden J, et al. Five-year prognostic significance of global longitudinal strain in individuals with a hypertrophic cardiomyopathy gene mutation without hypertrophic changes. J Am Soc Echocardiogr 27 (2019): 117-126.
- Bhattacharya M, Lu DY, Ventoulis I, Greenland GV, Yalcin H, et al. Machine learning methods for identifying atrial fibrillation cases and their predictors in patients with hypertrophic cardiomyopathy: the HCM-AF-Risk Model. J Am Heart Assoc 3 (2021): 801-813.
- Darwish RK, Haghighi A, Seliem ZS, El-Saiedi SA, Radwan NH, et al. Genetic study of pediatric hypertrophic cardiomyopathy in Egypt. Egypt Heart J 30 (2020): 1910-1916.
- Magrì D, Mastromarino V, Gallo G, Zachara E, Re F, et al. Risk stratification in hypertrophic cardiomyopathy: insights from genetic analysis and cardiopulmonary exercise testing. ESC Heart Fail 9 (2020): 1636.
- Miron A, Lafreniere-Roula M, Steve Fan CP, Armstrong KR, Dragulescu A, et al. A validated model for sudden cardiac death risk prediction in pediatric hypertrophic cardiomyopathy. Circulation 142 (2020): 217-229.
- Velicki L, Jakovljevic DG, Preveden A, Golubovic M, Bjelobrk M, et al. Genetic determinants of clinical phenotype in hypertrophic cardiomyopathy. ESC Heart Fail 20 (2020): 1-10.
- Phan PD, Tran VT, Pham MN, Mai AT, An DT, et al. Electrocardiographic and genetic characteristics in first degree relatives of hypertrophic cardiomyopathy probands: a descriptive cross-sectional study from Vietnam. J Cardiovasc Dev Dis 13 (2024): 20480040231220100.
- Xie CX, Machado GC. Clinimetrics: Grading of recommendations, assessment, development and evaluation (GRADE). J Physiother 67 (2021): 66.
- Brennan SE, Johnston RV. Research Note: Interpreting findings of a systematic review using GRADE methods. J Physiother 69 (2023): 198-202.
- Afonso J, Ramirez-Campillo R, Clemente FM, Büttner FC, Andrade R. The perils of misinterpreting and misusing “publication bias” in meta-analyses: an education review on funnel plot-based methods. Sports Med 54 (2024): 257-269.
- Favorito LA. Systematic review and meta-analysis in urology: how to interpret the forest plot. Int Braz J Urol 49 (2023): 775-778.
- Zhang Z, Kossmeier M, Tran US, Voracek M, Zhang H. Rainforest plots for the presentation of patient-subgroup analysis in clinical trials. Ann Transl Med 5 (2017): 485.
- Feczko E, Fair DA. Methods and challenges for assessing heterogeneity. Biol Psychiatry 88 (2020): 9-17.
- McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non–small-cell lung cancer. JAMA Oncol 2 (2016): 46-54.
- Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health 7 (2019): 192-198.
- Wang X, Piantadosi S, Le-Rademacher J, Mandrekar SJ. Statistical considerations for subgroup analyses. J Thorac Oncol 16 (2021): 375-380.
- Georgiopoulos G, Figliozzi S, Pateras K, Nicoli F, BampatsiasD, et al. Comparison of demographic, clinical, biochemical, and imaging findings in hypertrophic cardiomyopathy prognosis: a network meta-analysis. Heart Fail 11 (2023): 30-41.
- Amr A, Koelemen J, Reich C, Sedaghat-Hamedani F, Kayvanpour E, et al. Improving sudden cardiac death risk stratification in hypertrophic cardiomyopathy using established clinical variables and genetic information. Clin Res Cardiol 113 (2024): 728-736.
- Mistrulli R, Ferrera A, Salerno L, Vannini F, Guida L, et al. Cardiomyopathy and sudden cardiac death: bridging clinical practice with cutting-edge research. ESC Heart Fail 12 (2024): 1602.
- Bonaventura J, Rowin EJ, Chan RH, Chin MT, Puchnerova V, et al. Relationship between genotype status and clinical outcome in hypertrophic cardiomyopathy. J Am Heart Assoc 13 (2024): e033565.
- O’Mahony C, Jichi F, Ommen SR, Christiaans I, Arbustini E, et al. International external validation study of the 2014 European Society of Cardiology guidelines on sudden cardiac death prevention in hypertrophic cardiomyopathy (EVIDENCE-HCM). Circulation 137 (2018): 1015-1023.
- Santos EdS, Kuhn GdC, de Almeida AGC, João VictorAndrade Pimentel, Newton VitalFigueiredo Neto, et al. Genetic, clinical, and sociodemographic profile in individuals with diagnosis or family history of hypertrophic cardiomyopathy: insights from a prospective cohort. medRxiv (2025): 2025.2001.2024.25321106.



 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks