The Estrogen Receptor Beta Agonist Liquiritigenin Enhances Growth Inhibition of TNBC by the Cholesterol Biosynthesis Inhibitor RO 48-8071
Yayun Liang1, Benford Mafuvadze2, Salman M. Hyder1*
1Department of Biomedical Sciences, University of Missouri, MO 65211, Columbia, USA
2Alabama College of Osteopathic Medicine, Dothan, Alabama, USA
*Corresponding Author: Dr. Salman M. Hyder, Department of Biomedical Sciences, University of Missouri, MO 65211, Columbia, USA. Email: hyders@missouri.edu
Received: 11 June 2025; Accepted: 18 June 2025; Published: 26 June 2025
Article Information
Citation: Yayun Liang, Benford Mafuvadze, Salman M. Hyder. The Estrogen Receptor Beta Agonist Liquiritigenin Enhances Growth Inhibition of TNBC by the Cholesterol Biosynthesis Inhibitor RO 48-8071. Journal of Cancer Science and Clinical Therapeutics. 9 (2025): 87-96.
View / Download Pdf Share at FacebookAbstract
Purpose: Since many human TNBC develop resistance to aggressive forms of chemotherapy, our goal was to identify novel, less toxic treatment strategies that prevent drug resistance in TNBC. Previously, we established that RO 48-8071 ([4’-[6-(Allylmethylamino)hexyloxy]-4- bromo-2’-fluorobenzophenone fumarate] [RO], a small-molecule inhibitor of oxidosqualene cyclase (OSC), a key enzyme in the biosynthesis of cholesterol, inhibited TNBC growth of TNBC and induced tumor suppressor ERβ in TNBC cells. Consequently, we studied the effects of RO, together with liquiritigenin (LQ), a naturally occurring plant product that is an ERβ agonist, on TNBC progression.
Methods: Sulforhodamine B assays were used to measure viability of cultured MDA-MB-231 and BT-20 human TNBC cells in the presence of either RO and LQ alone or in combination. Tumor xenografts in nude mice permitted the evaluation of tumor growth following treatment with RO, LQ or a combination of RO and LQ. Estrogen receptor expression, apoptosis and levels of angiogenesis markers in tumor-xenograft tissues were assessed immunohistochemically.
Results: Both RO and LQ significantly reduced the viability of MDAMB- 231 and BT-20 TNBC cells in vitro. RO + LQ treatment reduced cell viability to a greater extent than treatment with either individual agent. Administration of RO, LQ, and a combination of RO and LQ significantly inhibited the growth of MDA-MB-231 tumor xenografts in vivo. RO, LQ, and RO + LQ increased ERβ expression in vivo and significantly reduced the expression of angiogenesis markers. Both RO and LQ increased apoptosis in tumor xenografts; a combination of RO + LQ significantly enhanced apoptosis compared with levels observed in response to a single agent.
Conclusion: The ERβ ligand LQ significantly enhanced RO-mediated inhibition of TNBC cell viability in vitro, while also significantly increasing the inhibitory effects of RO on the growth of tumor xenografts in vivo. The anti-tumor properties of RO may in part be due to an offtarget effect that increases ERβ which may then interact with LQ to promote anti-proliferative effects. Anti-tumor effects included inhibition of angiogenesis and induction of apoptosis. A combination of RO and LQ may have potential as a novel treatment strategy against TNBC.
Keywords
Triple-negative breast cancer; Tumor growth; Cholesterol biosynthesis inhibitors; Estrogen receptor; Liquiritigenin; Combination therapy
Article Details
Abbreviations: ANOVA: Analysis of Variance; ER: Estrogen Receptor; FBS: Fetal Bovine Serum; ip: Intraperitoneal; iv: Intravenous; LQ: Liquiritigenin: OSC: Oxidosqualene Cyclase; PBS: Phosphate-BufferedS; RO: RO 48-8071 ([4’-[6-(Allylmethylamino)hexyloxy]-4-bromo-2’-fluorobenzophenone fumarate]); sc: Subcutaneous; SEM: Standard Error of the Mean; SRB: Sulforhodamine B; TNBC: Triple-Negative Breast Cancer; TUNEL: Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling; VEGF: Vascular Endothelial Growth Factor
1. Introduction
Triple-negative breast cancers (TNBC) represent approximately 15-20% of all detected breast cancers. TNBC lack ER, PR and Her2neu, proteins that are commonly targeted in breast cancer therapies [1,2]. Women who suffer from TNBC have limited treatment options, are administered toxic chemotherapeutic agents and have poor prognosis due to the emergence of drug-resistant cells that lead to tumor metastasis. New non-toxic therapeutic strategies to control TNBC progression and prevent metastasis are urgently needed. Whereas the progression of hormone-dependent breast tumors depends on the presence of estrogen receptor (ERα) [3,4], TNBC lack both hormone receptors and Her-2-neu, making targeted therapies difficult. As a consequence, those afflicted with TNBC must be treated with high-dose chemotherapy, leading to a number of undesirable side-effects and, ultimately, resistance to therapy. Drug-resistant tumors proliferate more aggressively than the drug-sensitive tumors from which they arose, and go on to metastasize [5,6], resulting in poorer outcomes.
RO 48-8071 ([4’-[6-(Allylmethylamino)hexyloxy]-4-bromo-2’-fluorobenzophenone fumarate] (RO) [7-9] is a small-molecule cholesterol biosynthesis inhibitor that suppresses oxidosqualene cyclase (OSC), an enzyme that acts downstream of HMG-CoA reductase to convert 2, 3-monoepoxysqualene to lanosterol (a key step in the biosynthesis of cholesterol) [7-9]. We previously examined the effects of RO on hormone-dependent breast cancer and found that it suppressed the growth of hormone-dependent breast tumors as well as TNBC, promoted degradation of ERβ, and elevated levels of ERβ [10,11] which is known to suppress breast-cancer progression and is now classified as a tumor suppressor [12]. Importantly, RO has also been shown to effectively suppress angiogenesis in many other types of cancer [13]. Based on our observations that RO increases ERβ levels, we hypothesized that RO might also act in concert with ligands that interact with ERβ, increasing its therapeutic potential to regulate TNBC tumor progression.
Many of the naturally occurring ERβ-interacting ligands are derived from plants; therefore, their use in combination with RO is an attractive approach because it avoids the undesirable toxicity associated with many synthetic chemotherapeutic compounds in common use. In the present report, we describe the effects of RO alone and in combination with a plant-derived ERβ-interacting ligand, liquiritigenin (LQ) [14-16], on TNBC cells (MDA-MB-231 and BT-20) and tumors derived from these cells. In human MDA-MB-231 and BT-20 cells, we found that RO or LQ alone significantly inhibited tumor growth in vivo, and that a combination of RO and LQ enhanced the growth inhibition observed when RO was administered alone in xenografts derived from MDA-MB-231 cells that represent an aggressive type of TNBC. Mechanistically, we determined that RO or LQ alone or in combination induced ERβ expression in tumor-xenograft cells. Moreover, we found that RO or LQ alone induced apoptosis and reduced angiogenesis-marker expression in tumor-xenograft cells, and that a combination of RO and LQ enhanced the effects observed using RO alone. These potent anti-tumor properties of RO and LQ, particularly when used together, suggest that the RO + LQ combination has significant potential as a means by which to clinically manage TNBC.
2. Materials and Methods
2.1 Cell lines and culture
MDA-MB-231 and BT-20, human TNBC cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA) and grown in phenol red-free DMEM/F12 medium (Invitrogen Corporation & Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA).
2.2 Reagents
RO 48-8071 was purchased from Sigma-Aldrich. LQ was obtained from Tocris BioScience (Bristol, United Kingdom). RO was dissolved in phosphate-buffered saline (PBS) and LQ was dissolved in DMSO for in vitro studies and in 50% DMSO/15% ethanol/35% PBS for in vivo studies.
2.3 Cell viability assay
The sulforhodamine B (SRB) assay [17] was used to measure cell viability, as we have previously described [18].
2.4 In vivo breast tumor-xenograft studies
All animal experiments were approved by the Institutional Review Committee at the University of Missouri. Female athymic nude mice (nu/nu, Foxn1), 5- to 6-weeks-old and weighing 20–22 g, were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN, USA). Mice were inoculated with MDA-MB-231 cells as we have previously described [19]. Tumor volumes were measured as described previously [19], and drug treatment was started when average tumor volumes reached approximately 90-100 mm3. Mice were treated with 10 mg/kg RO by intravenous (iv) injection in a 0.1 ml solution into the tail vein, and LQ was given via intraperitoneal (ip) injection at 20 mg/kg. Combination group were given both RO and LQ. Treatment was daily for the first three days, followed by an injection every other day for 10 additional treatments and then a final injection 16 h prior to sacrifice [20]. Thus, a total of 14 treatments were administered. Control mice received the same volume of PBS or LQ dilution cocktail on the same schedule. Animal weight and tumor volume were assessed twice weekly throughout the study.
2.5 Immunohistochemical studies
Tumors were collected following the last injection and processed for immunohistochemical analysis of ERβ and ERβ as described previously [20,21]. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays, as well as vascular endothelial growth factor (VEGF) and CD31 assays, were performed as previously described [22]. Quantitation of immunolabeled signal was achieved using a morphometric analysis program (Fovea Pro 3.0, Reindeer Graphics), on images photographed at 20X magnification as described earlier [20,21]. For ER signal, four to five tumors/group were analyzed, and two to three representative sections were collected from each tumor. Results were expressed as area in square pixels.
Quantitation of blood vessels was accomplished by photographing tissue sections at 20X magnification from four to five tumors/group that had been stained immunohistochemically for CD31. The total number of vessels in these digital images was counted in 26 to 35 fields/group, with each field representing approximately 0.39 mm2. Vessel density was calculated as vessel number per field.
2.6 Statistical analysis
Differences between groups or among groups were tested using one-way analysis of variance (ANOVA) with repeated measures over time. The assumption of the ANOVA was examined, and a nonparametric measure based on ranks was used if needed. Values are reported as mean ± standard error of the mean (SEM). When ANOVA indicated a significant effect (F-ratio, P < 0.05), the Student-Newman-Keuls multi-range test was used to compare the means of the individual groups. Statistical analyses were conducted using SigmaStat software, version 15. For immunohistochemical analysis, data were analyzed using Kruskal-Wallis ANOVA, followed by Tukey’s procedure as a posthoc test. For all comparisons, P < 0.05 was regarded as statistically significant.
3. Results
3.1 RO and LQ reduce TNBC cell viability in vitro
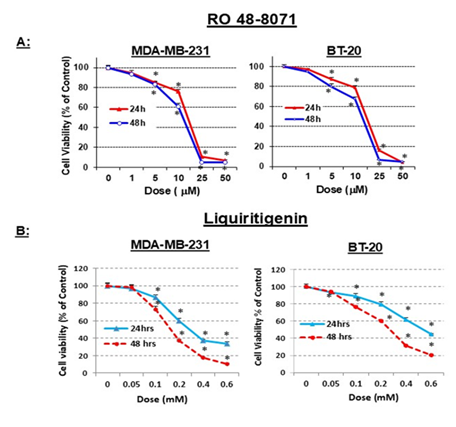
Both MDA-MB-231 and BT-20 TNBC cells were equally sensitive to RO (IC50 of 10-15 µM) after 48 h treatment (Figure 1A). LQ is a highly selective, naturally occurring ERβ agonist. Because we have previously shown that RO induces ERβ [10] and demonstrated this to be the case in TNBC cells [20], we examined whether LQ has the potential to enhance the inhibitory effects of RO in TNBC cells. We first tested whether LQ alone inhibits TNBC cells. Treatment of cells with LQ for 24 or 48 h significantly reduced cell viability compared with control cells (Figure 1B). After 48 h treatment, both MDA-MB-231 and BT-20 cells exhibited LQ-mediated loss of viability; LQ was more potent against MDA-MB-231 than BT-20 cells (IC50 0.15 mM vs 0.3 mM, respectively).
Figure 1: LQ reduces Triple Negative Breast-Cancer (TNBC) cell viability in vitro. (A): TNBC cells (MDA-MB-231: 4 ´103/well; BT-20: 5 ´ 103/well) were seeded in 96-well plates and incubated with pharmacological concentrations of RO for 24 or 48 h in DMEM/F12 + 5% FBS. (B) TNBC cells were incubated with pharmacological concentrations of LQ (mM) for 24 h or 48 h in DMEM/F12 + 5% FBS. Control cells were incubated with vehicle alone. Cell viability was determined by SRB assay. Values represent means ± SEM (n=6). * Significantly different from control (set at 100%) (P < 0.05 using ANOVA).
In a previous study we demonstrated that no differences in sensitivity of cells to LQ were observed between cells cultured in medium containing 5% FBS or 5% DCC-stripped FBS for 24 h [20], suggesting that reduction of cell viability by LQ was independent of the serum used, and that the 5% FBS used was likely devoid of any ERβ ligand that might mask the effects of LQ. Furthermore, these studies established that LQ concentrations between 0.1 and 0.3 mM, which demonstrate 20–50% growth inhibition of TNBC cells, would be appropriate to use in combination with RO to observe any additive or synergistic effects.
3.2 LQ enhances RO-mediated reduction in TNBC cell viability in vitro
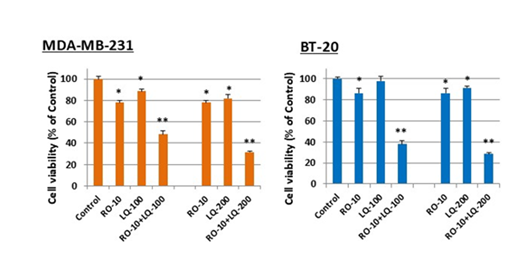
We next treated TNBC cells with RO alone or with LQ to determine whether a combination of the two agents might cause an even greater reduction in cell viability compared with individual treatments. Cells were exposed to RO first to enhance levels of ERβ. RO concentrations (5 or 10 µM) were selected, based on the present study and from observations previously published, which showed that these concentrations had sub-optimal effects in reducing cell viability [20]. LQ concentrations were chosen from the studies on cell viability reported in Figure 1 (0.1–0.3 mM). Both MDA-MB-231 and BT-20 cells were treated with RO alone, LQ alone, or RO combined with LQ. At all concentrations examined, a combination of RO and LQ significantly reduced the viability of both TNBC cell types compared with cells treated with either RO or LQ alone; data with 10 µM RO and LQ at 100- and 200 mM is shown (Figure 2). Cell viability was reduced by a combination of RO and LQ in a largely synergistic manner compared with RO or LQ alone.
Figure 2: LQ enhances RO-mediated reduction in TNBC cell viability in vitro. TNBC cells (MDA-MB-231: 4 ´103/well; BT-20: 5 ´ 103/well) were seeded in 96-well plates overnight in DMEM/F12 + 10% FBS. After washing and replacement of medium with DMEM/F12 + 5% FBS, cells were incubated with 10 mM RO alone, 0.1, 0.2, or 0.3 mM LQ (Control) alone, or combinations of these concentrations of RO and LQ for 24 h. Control cells were incubated with vehicle alone (Con). Cell viability was determined by SRB assay. Values represent means ± SEM (n=6). * Significantly different from control (set at 100%), and ** Significantly different from RO and LQ alone (P < 0.05 using ANOVA).
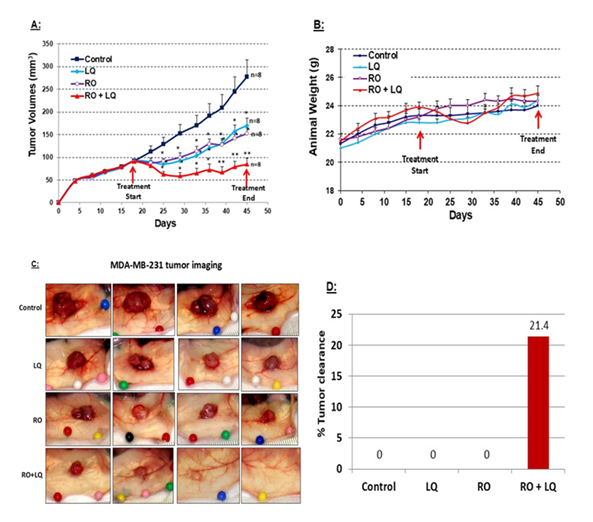
Figure 3: LQ enhances RO-mediated inhibition of TNBC MDA-MB-231 tumor-xenograft growth in vivo. Six-week-old nude mice were injected with TNBC MDA-MB-231 cells (5 ´ 106 in 0.15 ml of Matrigel: DMEM/F12 (4:1; [v/v]) in the flank, both flanks for each mouse. When average tumor volumes reached approximately 90-100 mm3, animals were treated over time with RO (10 mg/kg iv), LQ (20 mg/kg ip), or RO (10 mg/kg iv) + LQ (20 mg/kg ip), using an administration protocol described in the Methods. Control animals were treated with the same volume of vehicle. Tumors were collected on day 45 after MDA-MB-231 cell inoculation. (A) Tumor volumes were monitored throughout the experiment. Values represent means ± SEM (n=8 animals; please note that the curve was constructed with 14 tumors instead of 16 in each group since, due to technical difficulties with tumor volume measurements, 2 tumors were removed from each group). * Significantly different from control, ** Significantly different from RO and LQ alone groups (P < 0.05 using ANOVA). (B) Animal weight was monitored throughout the experiment. Values represent means ± SEM (n=8 animals). (C) Representative tumors in situ from each treatment group at the time of tumor collection. (D) Percentage of tumors cleared at the time of tumor collection for each treatment group.
3.3 LQ enhances RO-mediated inhibition of MDA-MB-231 tumor-xenograft growth in vivo
MDA-MB-231 is an aggressive TNBC used in many pre-clinical studies to identify potential methods of therapy. Using MDA-MB-231 cells we determined whether RO and LQ suppress the growth of MDA-MB-231 tumor xenografts in nude mice, and whether a combination of the two might act synergistically to more effectively suppress tumor growth in a manner consistent with their in vitro effects on cell viability. MDA-MB-231 cells were injected into both flanks of nude mice and tumors allowed to reach approximately 90-100 mm3. Animals were then separated into four groups and treated over time with vehicle control, LQ, RO, or RO + LQ, and tumor volumes monitored. Treatment continued until day 45, at which point animals were euthanized and tumors collected for further analysis. Treatment with RO or LQ significantly inhibited the growth of MDA-MB-231 tumors by approximately 45% compared with control-treated animals; treatment with RO + LQ was much more effective than treatment with a single agent and inhibited tumor growth by approximately 70% compared with control-treated animals. Most importantly, while tumor growth slowly resumed over time in animals treated with either RO or LQ, this was not the case with combination RO + LQ treatment (Figure 3A). Animals maintained their weight throughout all treatment regimens, demonstrating no measurable toxicity of either RO or LQ (Figure 3B). RO + LQ treatment eradicated 20 % of tumors, whereas treatment with RO or LQ alone did not eradicate tumors in the time frame that was tested (Figure 3C and D).
3.4 LQ and RO increase apoptosis in MDA-MB-231 tumor-xenograft cells in vivo
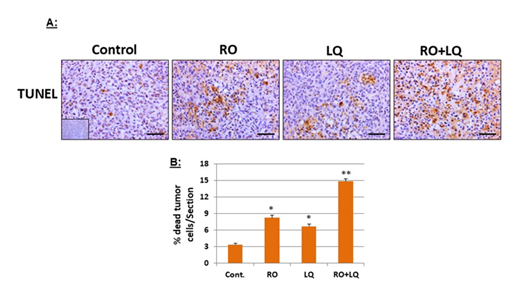
Using TUNEL assays to examine apoptosis in MDA-MB-231 xenograft tissues collected from animals treated over time with RO, LQ, or RO + LQ, we found that treatment with RO or LQ alone significantly induced in vivo apoptosis of MDA-MB-231 cells in xenografts compared with control-treated animals. A combination of RO and LQ significantly increased apoptosis compared with either agent alone (Figure 4), this effect being additive. These findings suggest that induction of apoptosis is a major mechanism by which these compounds suppress tumor growth.
Figure 4: LQ and RO increase apoptosis in MDA-MB-231 tumor-xenograft cells in vivo. Tumors collected at the endpoint of the MDA-MB-231 tumor-xenograft experiments presented in Figure 3 were subjected to TUNEL assays (A), then data was quantitated (B). Insets for representative micrographs represent negative controls. The mean values from 5-6 animal tumors with 30 sections for each group, which represent means ± SEM (n=30) are shown. Bars represent 50 mm. * Significantly different compared with control; ** Significantly different compared with all other groups (P < 0.05 using ANOVA).
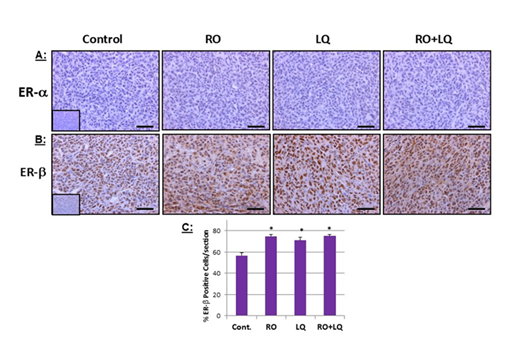
Figure 5: LQ and RO increase ERb expression in MDA-MB-231 tumor xenograft cells in vivo. Tumors collected at the endpoint of the MDA-MB-231 tumor-xenograft experiments presented in Figure 3 were processed for immunohistochemical analysis of ERa (A) and ERb (B), after which data was quantitated. Insets for representative micrographs represent negative controls. (A): MDA-MB-231 did not express ERa, hence all treatment groups showed negative expression. (B): RO increased ERb expression: mean values from 4-5 animal tumors with a total of 25-38 sections. Values shown are means ± SEM (n=25-38). Bars represent 50 mm. * Significantly different from control; ** Significantly different from all other groups (P < 0.05 using ANOVA).
3.5 LQ and RO induce ERβ expression in MDA-MB-231 tumor-xenograft cells in vivo
In previously published studies, we showed that RO effectively induces ERβ in hormone-dependent cells and in TNBC, both in vitro and in vivo [10,20]. When we analyzed MDA-MB-231 xenograft tissues collected from animals treated over time with RO, LQ, or RO + LQ, we found that both RO and LQ alone significantly induced ERβ expression in tumor-xenograft cells, compared with control-treated animals (Figure 5A and 5B) without influencing ERα levels (which are absent in TNBC cells). In tumor-xenograft cells from RO + LQ-treated animals, LQ significantly enhanced RO-mediated reduction of ERβ compared with RO treatment alone. Compared with control-treated animals, ERβ was also significantly induced in tumors from RO + LQ-treated animals, though the inclusion of LQ in this treatment regimen did not enhance induction of ERβ over that observed with RO alone (Figure 5C).
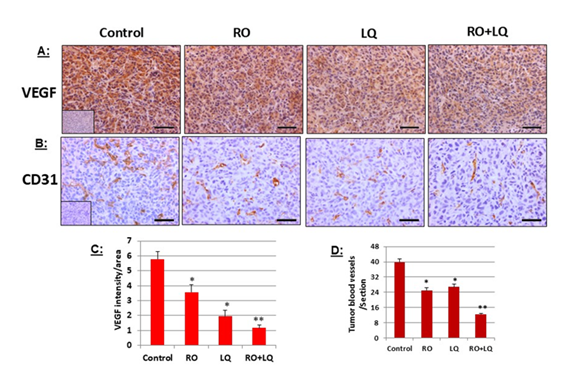
Figure 6: LQ and RO reduce angiogenesis-marker expression in MDA-MB-231 tumor-xenograft cells in vivo. Tumors collected at the endpoint of the MDA-MB-231 tumor-xenograft experiments presented in Figure 3 were processed for immunohistochemical analysis of VEGF (A) and CD31 (B), then data was quantitated. Insets for representative micrographs represent negative controls. (A): mean values from 5 animal tumors with 23-25 sections; (B): mean values from 5 animal tumors with 26-30 sections. Values represent means ± SEM. Bars represent 50 mm. * Significantly different from control; ** Significantly different from all other groups (P < 0.05 using ANOVA).
3.6 LQ and RO reduce angiogenesis-marker expression in MDA-MB-231 tumor-xenograft cells in vivo
Tumor growth depends on the pathologic formation of new blood vessels. Consequently, we analyzed two markers of angiogenesis, VEGF, and blood-vessel density, in MDA-MB-231 tumor-xenograft tissues to ascertain whether RO and LQ exert their anti-tumor effects in part by inhibiting angiogenesis. Compared with control-treated animals, treatment with RO or LQ alone significantly reduced VEGF expression (Figure 6). A combination of RO + LQ had an even greater effect than either agent alone, significantly reducing expression of VEGF compared with treatment with a single agent (Figure 6A). A similar effect was observed when tumor blood-vessel density was examined by CD31 immunohistochemical analysis; treatment with either RO or LQ significantly reduced blood-vessel density compared with control-treated animals, while combined treatment with RO + LQ significantly reduced blood-vessel density compared with either agent alone (Figure 6B). Figure 6C and D represents quantitation of data shown in Figure 6 A and B.
4. Discussion
Approximately 15% of human breast cancers belong to the TNBC type [1,2]. TNBC lack proteins that can be suppressed in a targeted approach to reduce tumor growth and are thus treated with toxic chemotherapeutic drugs to control the disease. In recent years, we have examined the effectiveness of a variety of compounds with low or no toxicity to control both hormone-dependent and TNBC tumor growth and found that the cholesterol biosynthesis inhibitor RO inhibited disease progression in both classes of breast cancer [11,20]. We found that in hormone-dependent breast cancer cells, RO downregulated both ERβ and progesterone receptor (PR), while simultaneously inducing the anti-proliferative protein ERβ. Similar effects were observed in TNBC with respect to ERβ induction [10,20]. ERβ is now known to function as a tumor suppressor [23-26]. Since LQ enhances RO-mediated inhibition of hormone-dependent breast cancer cell proliferation [20], we examined whether the naturally occurring compound might exert the same inhibitory effect on TNBC when administered in combination with RO. We did indeed observe that LQ greatly enhanced the growth-inhibitory properties of RO, both in vitro and in vivo when used against TNBC.
Both RO and LQ effectively suppressed the viability of MDA-MB-231 and BT-20 TNBC cells, with MDA-MB-231 cells exhibiting greater sensitivity to LQ. Breast-cancer cells express low levels of ERβ [27], for which LQ is an agonist. RO was administered to first enhance expression of ERβ, then cells were further treated with LQ. While LQ significantly reduced the viability of both breast-cancer cell lines, cells exposed to a combination of RO and LQ were affected to a greater extent. To ascertain whether the effects of the two agents were synergistic, both ligands were administered at sub-optimal levels. Our observations show that the inhibitory effects of RO and LQ on cell viability were indeed synergistic, suggesting that lower chemotherapeutic doses could be used in the clinical setting.
To further explore the use of a combination protocol, MDA-MB-231 cells were treated in vivo with RO and LQ and the inhibitory effects of mono- and combination therapy on tumor xenografts determined. Both RO and LQ alone significantly inhibited in vivo tumor growth. Treatment with RO + LQ elicited an even greater response; inhibition of tumor growth was significantly increased compared with either agent alone. During the period tested, RO and LQ alone did not cause tumor clearance, but when a combination of RO and LQ was administered, we did observe tumor clearance. These results suggest that RO + LQ combination therapy could be a valuable means of treating TNBC.
In addition to inhibiting the growth of TNBC both in vitro and in vivo, RO and LQ modestly but significantly induced ERβ expression in tumor-xenograft tissues, an effect that was observed in response to RO or LQ alone, as well as with a combination of RO and LQ. Although the induction of ERβ previously observed in TNBC in the in vitro setting was significant [11], its upregulation was not as robust in vivo. This could be due to the timing of tumor collection. By the end of the study, at which point tumors were harvested, several days had elapsed since the initiation of treatment. We believe that those cells expressing elevated levels of ERβ had already undergone apoptosis and were consequently missed during subsequent analysis. This hypothesis requires confirmation by collecting tumors a few days after initial RO treatment (but before the endpoint used in the present studies) and assessing ERβ induction in those tissues. Because it appears that RO itself has no interaction with ERβ [28], the influence of increased ERβ in the presence of RO may drive the inhibition of tumor growth in a ligand-independent manner [27], whereas when LQ is present, inhibited tumor growth could occur via a ligand-dependent manner. In addition, the absence of ERβ in TNBC cells means that the brake on ERβ may no longer be present, a scenario which is known to inhibit tumor-cell proliferation [29]. This ratio has been shown to be an important predictor of cell growth; a high ratio of ERα/ERβ is proliferative, whereas increased expression of ERβ is associated with loss of tumor-cell proliferation [30-33]. In the present study, sustained levels of ERβ appear to facilitate the effects of LQ when used in combination with RO, enhancing the anti-proliferative properties of RO.
In the tumor-xenograft model, RO or LQ alone significantly induced tumor-cell apoptosis, while reducing significantly the expression of angiogenesis markers. The effects of combined RO + LQ treatment were significantly greater than those seen with individual treatment alone. Because ERβ is known to induce apoptosis, as well as reduce angiogenesis (which limits the nutrients required for tumor growth [32,34], we contend that ERβ plays a key role in multiple mechanisms that curtail tumor growth.
5. Conclusion
In summary, the data presented strongly suggest that, in addition to its ability to suppress cholesterol biosynthesis [35], the OSC inhibitor RO exerts a powerful anti-tumor effect in TNBC cells through an off-target induction of the anti-proliferative protein ERβ. The natural compound LQ also induced ERβ in TNBC cells and very effectively enhanced the anti-proliferative effects of RO, most likely by binding to elevated levels of Erβ induced by RO and thereby potentiating its anti-tumor effects. Previous data show that ERβ promotes some of the same anti-tumor effects seen in response to RO. We propose that ERβ is responsible for the reduced viability of breast-cancer cells observed in response to RO or LQ alone, or when administered in combination. We suggest, therefore, that combination therapy using OSC inhibitors (unlike statins, which inhibit cholesterol biosynthesis by targeting HMG-CoA reductase, and do not influence ERβ [20]), together with naturally occurring compounds with affinity for ERβ could prove beneficial as a means by which to suppress TNBC growth.
Acknowledgments
SMH is the Zalk Missouri Professor of Tumor Angiogenesis and this research was supported by funds endowed by Mrs. Thelma Zalk.
Conflicts of interest/Competing interests (include appropriate disclosures)
Authors do not have conflict of interest.
Ethics approval (include appropriate approvals or waivers)
All animal studies were approved by ACUC at the University of Missouri.
References
- Jie H, Ma W, Huang C. Diagnosis, Prognosis, and Treatment of Triple-Negative Breast Cancer: A Review. Breast Cancer (Dove Med Press) 17 (2025): 265-274.
- Chen Z, Liu Y, Lyu M, et al. Classifications of triple-negative breast cancer: insights and current therapeutic approaches. Cell Biosci 15 (2025): 13.
- Cordera F, Jordan VC. Steroid receptors and their role in the biology and control of breast cancer growth. Semin Oncol 33 (2006): 631-641
- D'Abreo N, Hindenburg AA. Sex hormone receptors in breast cancer. Vitam Horm 93 (2013): 99-133.
- Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9 (2009): 631-643
- Hiscox S, Morgan L, Green TP. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat 97 (2006): 263-274.
- Charlton-Menys V, Durrington PN. Squalene synthase inhibitors: clinical pharmacology and cholesterol-lowering potential. Drugs 67 (2007): 11-16.
- Staedler D, Chapuis-Bernasconi C, Dehmlow H, et al. Cytotoxic Effects of Combination of Oxidosqualene Cyclase Inhibitors with Atorvastatin in Human Cancer Cells. J Med Chem 55 (2012): 4990-5002.
- Thoma R, Schulz-Gasch T, D'Arcy B, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature 432 (2004): 118-122.
- Liang Y, Besch-Williford C, Aebi JD, et al. Cholesterol biosynthesis inhibitors as potent novel anti-cancer agents: suppression of hormone-dependent breast cancer by the oxidosqualene cyclase inhibitor RO 48-8071. Breast cancer research and treatment 146 (2014): 51-62.
- Liang Y, Hyder SM. Oxidosqualene cyclase Inhibitor RO 48-8071 increases functional ERβ levels in triple-negative breast cancer cells. Journal of Cancer Science and Clinical Therapeutics 8 (2024): 216-222.
- Mal R, Magner A, David J, et al. Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor. Frontiers in Oncology 10 (2020): 587386.
- Maione F, Oliaro-Bosso S, Meda C, et al. The cholesterol biosynthesis enzyme oxidosqualene cyclase is a new target to impair tumour angiogenesis and metastasis dissemination. Scientific Reports 5 (2015): 9054.
- Mersereau JE, Levy N, Staub RE, et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol 283 (2008): 49-57.
- Liu Y, Xie S, Wang Y, et al. Liquiritigenin inhibits tumor growth and vascularization in a mouse model of HeLa cells. Molecules 17 (2012): 7206-7216
- Kim YW, Zhao RJ, Park SJ, et al. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br J Pharmacol 154 (2008): 165-173.
- Rubinstein LV, Shoemaker RH, Paull KD, et al. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst 82 (1990): 1113-8.
- Liang Y, Besch-Williford C, Benakanakere I, et al. Re-activation of p53 pathway inhibits growth of hormone-dependent human breast cancer cells in vitro and in vivo. Int J Oncology 31 (2007): 777-784.
- Liang Y, Besch-Williford C, Benakanakere I, et al. Targeting mutant p53 protein and the tumor vasculature: an effective combination therapy for advanced breast tumors. Breast Cancer Res Treat 125 (2011): 407-420.
- Liang Y, Besch-Williford C, Hyder SM. The estrogen receptor beta agonist liquiritigenin enhances the inhibitory effects of the cholesterol biosynthesis inhibitor RO 48-8071 on hormone-dependent breast-cancer growth. Breast Cancer Res Treat192 (2022): 53-63.
- Mafuvadze B, Benakanakere I, López Pérez FR, et al. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Cancer Prev Res 4 (2011): 1316-1324.
- Liang Y, Besch-Williford C, Mafuvadze B, et al. Combined treatment with p53-activating drug APR-246 and a phosphatidylserine-targeting antibody, 2aG4, inhibits growth of human triple-negative breast cancer xenografts. Cancer Rep Rev 4 (2020): 1-10.
- Treeck O, Lattrich C, Springwald A, et al. Estrogen receptor beta exerts growth-inhibitory effects on human mammary epithelial cells. Breast Cancer Res Treat 120 (2010): 557-565.
- Paruthiyil S, Parmar H, Kerekatte V, et al. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 64 (2004): 423-428.
- Warner M, Gustafsson JA. The role of estrogen receptor beta (ERbeta) in malignant diseases—a new potential target for antiproliferative drugs in prevention and treatment of cancer. Biochem Biophys Res Commun 396 (2010): 63-66
- Deroo BJ, Buensuceso AV. Minireview: Estrogen receptor-beta: mechanistic insights from recent studies. Mol Endocrinol 24 (2010): 1703-1714.
- Yan S, Dey P, Ziegler Y, et al. Contrasting activities of estrogen receptor beta isoforms in triple negative breast cancer. Breast Cancer Research and Treatment 185 (2021): 281-292.
- Mafuvadze B, Liang Y, Hyder SM. Cholesterol synthesis inhibitor RO 48-8071 suppresses transcriptional activity of human estrogen and androgen receptor. Oncology Reports 32 (2014): 1727-1733.
- Sotoca AM, van den Berg H, Vervoort J, et al. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells Toxicol Sci 105 (2008): 303-311.
- Lindberg MK, Movérare S, Skrtic S, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a "ying yang" relationship between ER-alpha and ERbeta in mice. Mol Endocrinol 17 (2003): 203-208.
- Williams C, Edvardsson K, Lewandowski SA, et al. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene 27 (2008): 1019-1032.
- Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 11 (2011): 597-608.
- Madeira M, Mattar A, Logullo AF, et al. Estrogen receptor alpha/beta ratio and estrogen receptor beta as predictors of endocrine therapy responsiveness-a randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer. BMC Cancer 13 (2013): 425.
- Hartman J, Lindberg K, Morani A, et al. Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res 66 (2006): 11207-11213.
- Ding X, Zhang W, Li S, et al. The role of cholesterol metabolism in cancer. American Journal of Cancer Research 9 (2019): 219-227.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 74.74%
Acceptance Rate: 74.74%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 