Augmented Reality-Assisted Implant Positioning: A Novel Method for Real- Time Precision Placement
Javier Orozco Martínez1*, Tanya Fernández-Fernández1, Amaia Iribar-Zabala2,3, Elena Aguilera-Jiménez4,5, Carla de Gregorio-Bermejo 2,4, Alicia Pose-Díez-de-la-Lastra2,4, Susana Gómez de los Infantes Peña4,5, Borja Lara Galdón1, Javier Pascau2,4, Mónica García-Sevilla2,4, José Calvo-Haro1,2,4,5,6, Rubén Pérez Mañanes1,2,4,5,6
1Department of Orthopaedic Surgery and Traumatology. Hospital General Universitario Gregorio Marañón, Madrid, Spain
2Department of Bioengineering. Universidad Carlos III de Madrid, Leganés, Spain
3Digital Health and Biomedical Technologies, Vicomtech Foundation, Basque Research and Technology Alliance (BRTA), Donostia-San Sebastian, Spain
4Instituto de Investigación Sanitaria Gregorio Marañón. Madrid, Spain
5Advanced Planning and 3D Manufacturing Unit (UPAM3D). Hospital General Universitario Gregorio Marañón, Madrid, Spain
6Department of Surgery, Universidad Complutense de Madrid, Madrid, Spain
*Corresponding Author: Javier Orozco Martínez: Orthopaedic Surgery and Traumatology, University Hospital Gregorio Marañón, Madrid, Spain.
Received: 21 March 2025; Accepted: 01 April 2025; Published: 30 July 2025
Article Information
Citation: Javier Orozco Martínez, Tanya Fernández-Fernández, Amaia Iribar-Zabala, Elena Aguilera-Jiménez, Carla de Gregorio-Bermejo, Alicia Pose-Díez-de-la-Lastra, Susana Gómez de los Infantes Peña, Borja Lara Galdón, Javier Pascau, Mónica García-Sevilla, José Calvo-Haro, Rubén Pérez Mañanes. Augmented Reality- Assisted Implant Positioning: A Novel Method for Real-Time Precision Placement. Journal of Surgery and Research. 8 (2025): 372-378.
View / Download Pdf Share at FacebookAbstract
Introduction: Accurate positioning of custom-made prostheses is critical for ensuring stability, functionality, and longevity. Conventional manual placement has limitations in precision and intraoperative flexibility. This study explores a novel augmented reality (AR)- based method that provides real-time feedback to enhance acetabular implant placement accuracy.
Materials and Methods: Custom-made prostheses were designed for ten cadaveric hemipelvis using 3D printing technologies. An AR-based navigation system, developed for the HoloLens 2, provided real-time visual feedback, integrating holographic target position projections and color-coded alignment feedback. Accuracy in prosthesis placement (angular and distance errors), AR marker-associated errors, and procedural time were evaluated.
Results: The mean angular error for prosthesis placement was 1.70° (95% CI: 0.99°–2.41°), ranging from 0.24° to 3.60°. The mean distance error was 1.75 mm (95% CI: 1.18–2.32 mm). AR marker-associated errors included a mean translational error of 1.07 mm and a rotational error of 0.86°. The AR-guided placement process had an average execution time of 56 seconds.
Conclusions: This study presents a novel AR-assisted guidance method that enables high-precision prosthesis placement. The results highlight its potential to enhance accuracy and efficiency in complex surgical workflows, supporting its integration into future orthopaedic procedures.
Keywords
<p>Augmented reality (AR); Custom-made implant; 3D HoloLens; Real-time; Computer assisted surgery (CAS); Orthopaedic surgery; Positioning; Patient-specific guidance</p>
Article Details
Introduction
Materials and Methods
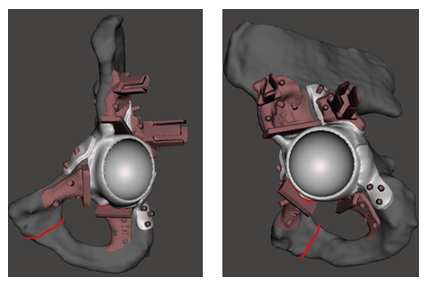
The process began with acquiring a CT scan of 10 cadaveric specimens and the hemipelvis bone was manually segmented using 3D Slicer [1]. Personalized prostheses were subsequently designed to fit previously planned periacetabular resections by expert clinicians (Figure 1).
The design of the PSIs was carried out using 3-matic software (Materialise, Belgium). For the prostheses design, an acetabular implant was created, maintaining the same centre of rotation as the patient’s acetabulum and incorporating three anchors for screw fixation to the ilium, ischium, and pubis (Figure 2). A removable socket was added to the prosthesis for the placement of an AR marker.
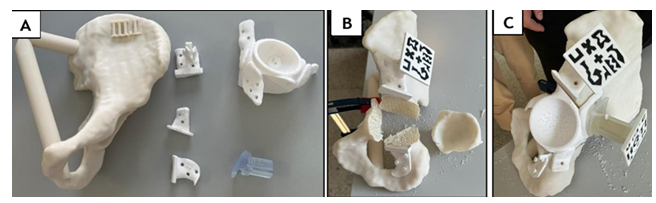
Figure 2: (A) Materials used in the study: Cadaveric hemipelvis biomodel fabricated from acrylonitrile styrene acrylate (ASA); patient-specific cutting guides (PSIs) and custom-made prosthesis, both 3D printed in rigid radiopaque 10k resin; AR markers printed in black and white polylactic acid (PLA) filament; and a removable AR marker socket designed for intraoperative prosthesis tracking and alignment. (B) Planned periacetabular resection. (C) Prosthesis positioned and secured.
The resection planes were used as a reference to design three patient-specific cutting guides: supraacetabular, ischial, and symphysial. A socket was also incorporated into the supraacetabular PSI to enable the placement of another AR marker, which served as the reference.
All components were 3D-printed using various materials and printers. The healthy portion of the bone was fabricated in acrylonitrile styrene acrylate (ASA) material, while the AR markers were printed using black and white polylactic acid (PLA) filament. The PSIs and the personalized prosthesis were manufactured in rigid radiopaque 10k resin. The choice of materials for each component was strategically made to facilitate their segmentation in a CT scan following the experiment for subsequent analysis.
For navigation, an AR application for the HoloLens 2 (Microsoft Corporation, WA, U.S.A.) HMD was developed to support navigation throughout the entire workflow of prosthesis placement [2].
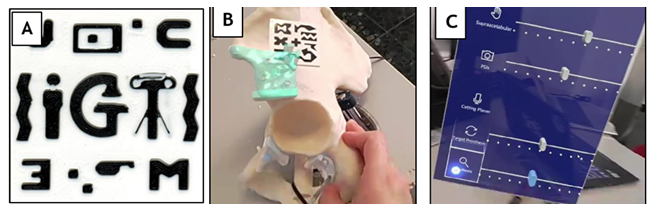
The application incorporates a hand menu with features such as on/off buttons for visualizing the prosthesis relative to the marker located in the supraacetabular region and sliders to adjust object transparency. The prosthesis visualization can be activated to display the model in semi-transparent white, enabling the user to see the prosthesis's target position and use it as a guide (Figure 3).
Figure 3: (A) Fiducial marker designed for AR detection. (B) Detection of the AR marker and precise overlay of the patient’s hemipelvis hologram onto the phantom. (C) Custom-designed menu integrated into our software, enabling the user to select display features (bone, target prosthesis, and prosthesis) and adjust transparency levels for enhanced visualization. Captured images through the HMDs (AR).
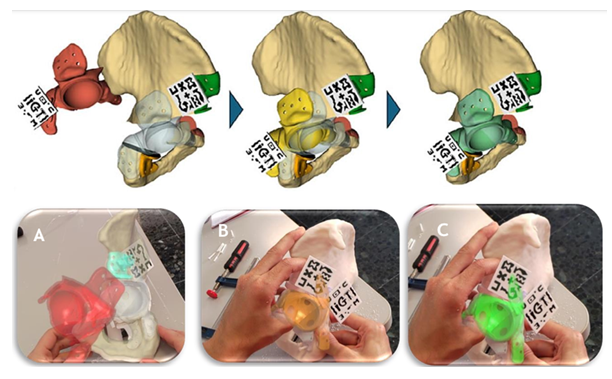
Additionally, a second marker placed on the prosthesis facilitates tracking its position. As the prosthesis moves closer to the target position, the marker's colour changes progressively from red to yellow and finally to green, indicating that an acceptable position has been achieved (Figure 4).
Figure 4: Prosthesis guidance system using a colour-coded feedback mechanism. As the prosthesis moves closer to the target position, the marker's colour changes progressively from red to yellow and finally to green, indicating that an acceptable position has been achieved. (A) Red indicates an incorrect prosthesis position with an error exceeding 7.5mm. (B) The prosthesis fits within the osteotomies but has an error of up to 7.5 mm from the planned ideal position, making it acceptable but not optimal. (C) Green signifies the optimal planned position, with an error below 2.5 mm. Captured images through the HMDs (AR).
The colour green indicates a minor positioning error of ±2.5 mm, the colour orange represents an error of up to ±7.5 mm, and the colour red signifies an error exceeding this distance.
Following the design and fabrication phases, the experiment was carried out by two expert clinicians (Figure 5).
The process began with the manual placement of the supraacetabular AR marker, which facilitated the hologram visualization on the HoloLens 2. An acetabular osteotomy was performed using PSIs. Subsequently, an AR marker was positioned on the prosthesis to enable its visualization, facilitating placement based on the holographic projection of the target position onto the phantom. Colour feedback on the implant hologram provided further guidance, indicating the correctness of the positioning.
Finally, a postoperative CT scan and further image segmentation were performed on all phantoms for analysis purposes. The analysis focused on assessing the placement and orientation of the prosthesis, as well as quantifying the error introduced by the final positioning of the AR marker. This workflow will be maintained for subsequent phases of the study.
To analyse the results, three metrics were evaluated: implant placement accuracy, the error introduced by the AR marker and the time required to complete each task.
The evaluation of prosthesis placement focused on two primary aspects: the position and orientation of the acetabular component, and the fitting of the prosthesis within the pelvis, assessed through plane comparison. This comparison provided the deviation in both rotation and maximum distance. The position of the acetabular component was determined by identifying its centre and comparing it with the one defined during the planning phase. For the orientation of the acetabulum, its horizontality or verticality was assessed in the coronal plane. This approach was based on the methodology outlined by Iribar Zabala et al., demonstrating its efficacy in measuring deviations in AR-assisted procedures [3].
Ideally, this angle should fall within the range of 40° to 50°. Additionally, a slight anteversion, measured relative to the sagittal plane, is desirable, typically ranging between 15° and 20°. The error is reported in relative values, calculated as the difference between the planned angles and the angles obtained during the procedure.
On the other hand, the error associated with the AR marker was computed by registering the preoperative CT with the postoperative CT. The origin of both segmentations’ coordinate systems was placed at the centre of the supraacetabular marker, where x and y axes are in the plane of the marker, and the z axis is the normal vector pointing outward from the centre.
Finally, the time spent for the different procedure tasks was measured: supraacetabular PSI placement, prosthesis placement and prosthesis fixation.
Results
The results for ten phantoms from the different cadavers are shown in the following sections. The phantoms are named in Roman numbers according to the cadaver specimen identification.
Implant placement accuracy
The acetabular angular error had an average value of 1.70º (95% CI: 0.99º - 2.41º) with a standard deviation of 1.00º. The values ranged from a minimum of 0.24º in Case VII to a maximum of 3.60º in Case II.
On the other hand, the distance error relative to the planned acetabular centre of rotation had a mean value of 1.75 mm (95% CI: 1.18 mm - 2.32 mm), with a standard deviation of 0.80 mm. The values ranged from 0.86 mm in Case V to 3.45 mm in Case IX (Table 1).
|
Case |
Angular error (º) |
Distance error (mm) |
|
I |
1.27 |
1.17 |
|
II |
3.6 |
1.2 |
|
III |
0.41 |
1.81 |
|
IV |
1.45 |
2.53 |
|
V |
1.39 |
0.86 |
|
VI |
2.46 |
1.43 |
|
VII |
0.24 |
2.17 |
|
VIII |
2.2 |
1.04 |
|
IX |
2.28 |
3.45 |
|
X |
1.68 |
1.88 |
|
Mean |
1.7 |
1.75 |
|
Deviation |
1 |
0.8 |
Table 1: Acetabular placement accuracy. Angular and distance errors.
When analysing prosthesis placement accuracy concerning contact surfaces (supraacetabular, symphysial and ischial), all regions exhibited similar results, with a mean angular error of 1.64° ± 1.82° (Table 2).
|
Case |
Prosthesis Contact Surface |
Angular error (°) |
|
I |
Supraacetabular |
0.55 |
|
Symphysial |
0.56 |
|
|
Ischial |
1.24 |
|
|
II |
Supraacetabular |
0.9 |
|
Symphysial |
5.43 |
|
|
Ischial |
5.04 |
|
|
III |
Supraacetabular |
0.39 |
|
Symphysial |
0.67 |
|
|
Ischial |
0.67 |
|
|
IV |
Supraacetabular |
2.21 |
|
Symphysial |
2.23 |
|
|
Ischial |
1.49 |
|
|
V |
Supraacetabular |
1.59 |
|
Symphysial |
1.07 |
|
|
Ischial |
0.94 |
|
|
VI |
Supraacetabular |
1.97 |
|
Symphysial |
1.32 |
|
|
Ischial |
2.15 |
|
|
VII |
Supraacetabular |
1.18 |
|
Symphysial |
0.45 |
|
|
Ischial |
1.17 |
|
|
VIII |
Supraacetabular |
3.08 |
|
Symphysial |
2.87 |
|
|
Ischial |
1.95 |
|
|
IX |
Supraacetabular |
0.54 |
|
Symphysial |
1.92 |
|
|
Ischial |
1.45 |
|
|
X |
Supraacetabular |
0.99 |
|
Symphysial |
1.88 |
|
|
Ischial |
1.27 |
|
|
Mean (95% CI) |
1.64 (± 1.82°) |
Table 2: Accuracy of prosthesis placement: angular errors across the supraacetabular, symphysial, and ischial contact surfaces.
The maximum angular error was observed in Case II at the pubic symphysis, with a deviation of 5.43°. The region with the lowest angular error was the supraacetabular surface, with a mean error of 1.34° ± 0.87°, followed by the ischium at 1.74° ± 1.24°, and the pubis at 1.84° ± 1.49°.
AR-marker associated error
Table 3 illustrates the errors associated with supraacetabular marker positioning, calculated by comparing the planned marker position with the postoperative CT scan registration of the positioned marker. The global translation error across the three axes (Tx, Ty, Tz) was 1.07 mm on average (95% CI: 0.82 mm - 1.32 mm, Std Dev: 0.68 mm), while the global rotational error (Rx, Ry, Rz) averaged 0.86° (95% CI: 0.55° - 1.17°, Std Dev: 0.84°), reflecting high precision in both translational and angular positioning. Overall, the values remain below 1 mm for translation and 1° for rotation.
|
Marker |
Tx (mm) |
Ty (mm) |
Tz (mm) |
Rx (°) |
Ry (°) |
Rz (°) |
|
I |
-1.31 |
1.74 |
-1.37 |
0.42 |
0.03 |
0.45 |
|
I |
-2.53 |
-1.56 |
-0.97 |
2.51 |
0.23 |
-0.4 |
|
III |
-2.13 |
0.31 |
-1.27 |
-0.3 |
-1.68 |
-1.11 |
|
IV |
-0.56 |
0.05 |
-0.5 |
-0.34 |
-0.23 |
0.25 |
|
V |
-1.28 |
0.58 |
0.29 |
0 |
0 |
0 |
|
VI |
-0.54 |
1.77 |
-0.8 |
-1.28 |
0.14 |
2.09 |
|
VII |
-1.12 |
0.02 |
-0.74 |
1.56 |
0.88 |
-3.34 |
|
VIII |
0.99 |
2.32 |
-0.05 |
-1.09 |
1.49 |
0.09 |
|
IX |
-1.06 |
1.18 |
-0.88 |
-1.3 |
-1.48 |
0.09 |
|
X |
-2.26 |
0.99 |
-0.92 |
-1.5 |
-1.07 |
-0.48 |
|
Mean (absolute value) |
1.38 |
1.05 |
0.78 |
1.03 |
0.72 |
0.83 |
|
Deviation |
0.7 |
0.8 |
0.41 |
0.77 |
0.67 |
1.08 |
Table 3: AR-marker associated errors: Translation and orientation deviations across the x, y, and z axes.
Procedure task times
Table 4 provides a summary of the time required for each task, presenting the mean times and standard deviations for both prosthesis placement and fixation. The AR-guided placement of the prosthesis had a mean duration of 56.64 seconds (95% CI: 21.37–91.90), while the fixation process averaged 132.32 seconds (95% CI: 98.89–165.74).
|
Time (s) |
Prosthesis AR-guided-placement |
Prosthesis fixation |
|
I |
150 |
90 |
|
II |
100 |
150 |
|
III |
175 |
148 |
|
IV |
53 |
77 |
|
V |
23 |
153 |
|
VI |
25 |
112 |
|
VII |
5 |
108 |
|
VIII |
120 |
182 |
|
IX |
125 |
240 |
|
X |
52 |
141 |
|
Mean time |
56.64 |
132.32 |
|
Deviation |
-57.95 |
-47.58 |
Table 4: Procedure task times [8].
Discussion
The findings of this study demonstrate that AR holds significant potential for enhancing the accuracy and efficiency of prostheses placement in orthopaedic oncology. These results align with previous research while offering new insights into the application of AR for guiding prosthesis placement, particularly in complex periacetabular resections.
Our study presents, for the first time, a method that enables real-time intraoperative assessment of implant positioning using augmented reality. Traditionally, implant placement accuracy could only be verified postoperatively through imaging studies, at which point any deviation from the planned position was irreversible. Recent studies developed an AR system that projects a hologram of the desired implant position onto the surgical site; however, they do not provide real-time feedback on placement errors.
With our system, the surgeon receives continuous real-time feedback on positional errors, allowing for immediate adjustments during surgery. The colour-coded guidance-green for minimal error, orange for moderate deviations, and red for unacceptable discrepancies-provides not only qualitative but also quantitative evaluation of positioning accuracy. This novel approach, which has not been previously described, significantly enhances surgical precision, reduces the risk of misplacement, and minimizes the need for corrective interventions, setting a new standard in implant placement techniques.
Beyond its use in orthopaedic surgery, AR-assisted implant guidance has been recently developed in other fields, with multiple research groups investigating various methods and reporting their outcomes.
Mai et al. [4] introduced an AR-based surgical navigation system in dentistry, allowing real-time visualization of the planned dental implant position over the surgical site. They reported average deviations of 0.90 mm (lateral), 0.78 mm (depth), and 1.18 mm (global) when using AR for dental implant guidance, achieving accuracy within the clinically acceptable safety zone. This approach therefore enhances precision compared to conventional manual and dynamic methods.
On the other hand, Lui et al. [5] applied AR projection technology to guide the placement of bone conduction devices in otologic surgery, achieving an improvement in the centre-to-centre distance between the planned and placed implant (from 9.0 mm to 1.9 mm).
Takács et al. evaluated multiple computer-assisted implant surgery (CAIS) methods, including dynamic navigation, static guidance and AR. AR demonstrated an average angular deviation of 3.73° in the orientation of dental implants [6]. The results indicate that AR-based guidance is comparable in precision to static systems, although it remains in the preclinical stage.
In our study, the mean distance error for prosthesis implantation was 1.75 mm, with a maximum deviation of 3.45 mm, underscoring a high degree of accuracy in prosthesis positioning, with the lowest error observed in the supraacetabular region (1.27 mm). In contrast, the ischium exhibited the greatest variability and the largest error, highlighting the challenges associated with anatomically complex regions.
In the field of orthopaedic surgery, Mendicino et al. documented an average error ranging from 2.19 mm to 4.72 mm when evaluating AR-assisted PSI placement in patient-specific pelvic phantoms [7]. Similarly, Lui et al. reported centre-to-centre distance errors of 1.9 mm in surgeries using AR, which were significantly lower than those in procedures without AR (9.0 mm) [6]. However, their study did not observe improvements in angular orientation
In our study, an average angular error of 1.70º with a maximum deviation of 3.60º was achieved, reflecting precise alignment.
Conversely, some studies have found no significant improvement in clinical outcomes for hip arthroplasty when the new centre of rotation was maintained within a 5 mm margin. However, these findings do not consider the increased complexity of oncologic reconstructive surgery or the challenges posed by custom-made implants, which present unique challenges [8,9].
In total hip arthroplasty (THA), AR has been shown to significantly improve the accuracy of acetabular component orientation [31]. AR has also been utilized for base plate component placement in reverse total shoulder arthroplasty, achieving an average angular error of 2.7° and a mean entry-point distance error of 2.3 mm [32]. These results indicate a precision comparable to computer-assisted optical navigation systems but at a lower cost, with significant potential for optimizing glenoid component placement in anatomically complex conditions.
The integration of devices such as Microsoft HoloLens enables a more efficient workflow, eliminating the need to shift focus to external monitors [5]. Regarding timing, AR-guided prosthesis placement in our study had a mean duration of 56.64 seconds. Although we did not compare it to placement without AR, Mendicino et al. suggest that users without visual references spend more time locating anatomical landmarks on exposed bone and perform the task with less confidence [1]. Their findings demonstrated a 30–40% reduction in the time required to position specific templates when using AR compared to relying solely on anatomical references. Similarly, Sun et al. observed reduced surgical times and improved efficiency with holographic guidance during complex procedures compared to traditional methods [10]. However, some authors have highlighted challenges with intraoperative anatomical registration, often requiring multiple attempts (2–3 per model) to achieve satisfactory results [11].
On the other hand, AR not only improves surgical accuracy but also offers notable economic benefits. As noted by Logishetty K [12] current orthopaedic simulators for training in arthroscopic and open surgeries often cost significantly more than the available AR platforms. Moreover, in another study, novice users trained with AR achieved better acetabular orientation in simulated procedures compared to those supervised by expert surgeons (1° ± 1° vs. 6° ± 4°) [13]. Furthermore, the integration of artificial intelligence into preoperative planning and automatic segmentation could further streamline workflows and reduce procedural errors [14-24].
In our study, the AR marker associated error demonstrated high precision in both translational and angular positioning. The global translation error across the three axes (Tx, Ty, Tz) averaged 1.07 mm while the global rotational error (Rx, Ry, Rz) averaged 0.86°. These results align with those reported by Lui et al., who observed projection accuracy of 1.7 ± 0.6 mm, and emphasizes the importance of robust optical marker systems to minimize translational and angular errors. This reinforces the reliability of AR-assisted registration as a tool for achieving precise prosthesis positioning [25-33].
Limitations and Areas for Improvement
Despite its promising potential, AR technology is not without limitations. Challenges include restricted battery life, physical discomfort during extended use, and a steep learning curve for surgeons who are unfamiliar with AR systems. To address these issues, iterative advancements in hardware design and the development of targeted training programs are essential. These improvements will enhance usability, reduce fatigue, and facilitate broader adoption among surgical professionals.
Conclusions
This study demonstrates the feasibility and efficacy of a novel AR-guidance method for improving the accuracy of prosthesis placement in a preliminary setting. Integrating AR into surgical workflows marks a significant advancement, enhancing precision and reducing errors. The acetabular placement showed an average angular error of 1.70º and a mean distance error of 1.75 mm from the planned centre of rotation.
This approach holds great potential for advancing complex oncologic reconstruction surgeries. Future work will focus on pre-clinical validation in cadaveric models, addressing current limitations, and expanding the clinical applications of AR technology to broader surgical contexts.
Acknowledgements
This work was funded through Projects CER-20231013 (Aid for Technological Centres Cervera), TED2021-132200B-I00, TED2021-129392B-I00, and PID2023-149604OB-I00, supported by the Ministerio de Ciencia e Innovación/AEI/10.13039/501100011033 and the European Union’s “NextGenerationEU”/PRTR initiative.
This study has been funded by Instituto de Salud Carlos III (ISCIII) through the Biomodels and Biobanks Platform and co-funded by the European Union (PT23/00116 to RPM).
Conflict of interest statement
The authors declare no conflicts of interest.
References
- Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30 (2012): 1323-1341.
- Moreta-Martinez R, Pose-Díez-de-la-Lastra A, Calvo-Haro JA, et al. Combining Augmented Reality and 3D Printing to Improve Surgical Workflows in Orthopedic Oncology: Smartphone Application and Clinical Evaluation. Sensors 21 (2021): 1370.
- Iribar-Zabala A, Fernández-Fernández T, Orozco-Martínez J, et al. AR-assisted surgery: Precision placement of patient specific hip implants based on 3D printed PSIs. Healthc Technol Lett 11 (2024): 402-410.
- Mai HN, Dam VV, Lee DH. Accuracy of Augmented Reality–Assisted Navigation in Dental Implant Surgery: Systematic Review and Meta-analysis. J Med Internet Res 25 (2023): e42040.
- Lui JT, Dahm V, Chen JM, et al. Using augmented reality to guide bone conduction device implantation. Sci Rep 13 (2023): 7182.
- Takács A, Hardi E, Cavalcante BGN, et al. Advancing accuracy in guided implant placement: A comprehensive meta-analysis: Meta-Analysis evaluation of the accuracy of available implant placement Methods. J 139 (2023): 104748.
- Mendicino AR, Condino S, Carbone M, et al. Augmented Reality as a Tool to Guide Patient-Specific Templates Placement in Pelvic Resections. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf (2022): 3481-3484.
- Morgan S, Amzallag N, Shaked O, et al. Accurate Restoration of the Center of Rotation of the Hip Joint Based on Preoperative Planning Is Not Associated with Improved Clinical Outcomes. Surgeries 4 (2023): 698-705.
- Traina F, De Fine M, Biondi F, et al. The influence of the centre of rotation on implant survival using a modular stem hip prosthesis. Int Orthop 33 (2009): 1513-1518.
- Sun P, Zhao Y, Men J, et al. Application of Virtual and Augmented Reality Technology in Hip Surgery: Systematic Review. J Med Internet Res 25 (2023): e37599.
- Kriechling P, Roner S, Liebmann F, et al. Augmented reality for base plate component placement in reverse total shoulder arthroplasty: a feasibility study. Arch Orthop Trauma Surg 141 (2021): 1447-1453.
- Logishetty K, Western L, Morgan R, et al. Can an Augmented Reality Headset Improve Accuracy of Acetabular Cup Orientation in Simulated THA? A Randomized Trial. Clin Orthop 477 (2019): 1190-1199.
- Verhey JT, Haglin JM, Verhey EM, et al. Virtual, augmented, and mixed reality applications in orthopedic surgery. Int J Med Robot Comput Assist Surg MRCAS 16 (2020): e2067.
- Mangano FG, Admakin O, Lerner H, et al. Artificial intelligence and augmented reality for guided implant surgery planning. A proof of concept J Dent 133 (2023): 104485.
- García-Sevilla M, Moreta-Martinez R, García-Mato D, et al. Augmented Reality as a Tool to Guide PSI Placement in Pelvic Tumor Resections. Sensors 21(2021): 7824
- Carrozzi A, Chylinski M, Heller J,et al. What’s Mine Is a Hologram? How Shared Augmented Reality Augments Psychological Ownership. J Interact Mark 48 (2019): 71-88
- Dolega-Dolegowski D, Proniewska K, Dolega-Dolegowska M,et al. Application of holography and augmented reality based technology to visualize the internal structure of the dental root – a proof of concept. Head Face Med 18 (2022): 12.
- Ackermann J, Liebmann F, Hoch A, et al. Augmented Reality Based Surgical Navigation of Complex Pelvic Osteotomies—A Feasibility Study on Cadavers. Appl Sci 11(2021): 1228
- Hoch A, Liebmann F, Farshad M,et al. Augmented reality-guided pelvic osteotomy of Ganz: feasibility in cadavers. Arch Orthop Trauma Surg 144 (2024): 1077-1089
- Pflugi S, Liu L, Ecker TM, et al. A cost-effective surgical navigation solution for periacetabular osteotomy (PAO) surgery. Int J Comput Assist Radiol Surg 11 (2016): 271-280
- Mediavilla-Santos L, García-Sevilla M, Calvo-Haro JA, et al. Validación de los modelos de impresión 3D paciente-específicos para cirugía ortopédica oncológica pélvica. Rev Esp Cir Ortopédica Traumatol 66 (2022): 403-409
- Ma L, Jiang W, Zhang B, et al. Augmented reality surgical navigation with accurate CBCT-patient registration for dental implant placement. Med Biol Eng Comput 57 (2019): 47-57
- Jiang W, Ma L, Zhang B, et al. Evaluation of the 3D Augmented Reality-Guided Intraoperative Positioning of Dental Implants in Edentulous Mandibular Models. Int J Oral Maxillofac Implants 33 (2018): 1219-1228
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 60 (2010): 277-300
- Mayerson JL, Wooldridge AN, Scharschmidt TJ. Pelvic resection: current concepts. J Am Acad Orthop Surg 22 (2014) :214-222
- Karaca MO, Özbek EA, Özyildiran M, et al. External and internal hemipelvectomy: A retrospective analysis of 68 cases. Jt Dis Relat Surg 33 (2022): 132-141
- Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 60 (1978): 731-746.
- Lewinnek GE, Lewis JL, Tarr R, et al. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 60 (2): 217-220.
- McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop 261 (1990): 159-170.
- Esposito CI, Gladnick BP, Lee Y yu, et al. Cup Position Alone Does Not Predict Risk of Dislocation after Hip Arthroplasty. J Arthroplasty 30 (2015): 109-113
- Jud L, Fotouhi J, Andronic O, et al. Applicability of augmented reality in orthopedic surgery - A systematic review. BMC Musculoskelet Disord 21 (2020): 103
- Bian D, Lin Z, Lu H, et al. The application of extended reality technology-assisted intraoperative navigation in orthopedic surgery. Front Surg 11 (2024): 1336703
- Bruschi A, Donati DM, Di Bella C. What to choose in bone tumour resections? Patient specific instrumentation versus surgical navigation: a systematic review. J Bone Oncol 42 (2023): 100503.



 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 