Functional and Immunological Monitoring of Immune-Reactive and Anti-Leukemic Cells (T, NK, CIK, B-Cells) arising in WB from AML, ALL, or CLL Patients under Treatment with Several Immunomodulatory Approaches
Xiaojia Feng*,1,2, Marianne Unterfrauner1,2, Sophia Bohlscheid1,2, Philipp Anand1,2, Lin Li1,2, Hazal Aslan Rejeski1,2, Anne Hartz1,2, Tobias Baudrexler1,2, Joudi Abdulmajid1,2, Anwesha Sinha1,2, Peter Bojko3, Doris Krämer4, Jörg Schmohl5, Christoph Schmid2,6, Giuliano Filippini Velázquez2,6 and Helga Schmetzer*,1,2
1Department of Medicine III, University Hospital of Munich, 81377 Munich, Germany
2Bavarian Center for Cancer Research (BZKF), 91054 Erlangen, Germany
3Department of Hematology and Oncology, Rotkreuzklinikum Munich, 80634 Munich, Germany
4Department of Hematology and Oncology, St.-Josefs-Hospital, 58097, Hagen, Germany
5Department of Hematology and Oncology, Diakonieklinikum Stuttgart, 70176 Stuttgart, Germany
6Department of Hematology and Oncology, University Hospital of Augsburg, 86156 Augsburg, Germany
*Corresponding Author: Xiaojia Feng, Department of Medicine III, University Hospital of Munich, 81377 Munich, Germany; Helga Schmetzer, Department of Medicine III, University Hospital of Munich, 81377 Munich, Germany;
Received: 22 August 2025; Accepted: 29 August 2025; Published: 25 November 2025
Article Information
Citation: Xiaojia Feng, Marianne Unterfrauner, Sophia Bohlscheid, Philipp Anand, Lin Li, Hazal Aslan Rejeski, Anne Hartz, Tobias Baudrexler, Joudi Abdulmajid, Anwesha Sinha, Peter Bojko, Doris Krämer, Jörg Schmohl, Christoph Schmid, Giuliano Filippini VelaÌzquez and Helga Schmetzer. Functional and Immunological Monitoring of Immune-Reactive and Anti-Leukemic Cells (T, NK, CIK, B-Cells) Arising in WB From AML, ALL, or CLL Patients Under Treatment with Several Immunomodulatory Approaches. Fortune Journal of Health Sciences. 8 (2025): 1089-1112.
View / Download Pdf Share at FacebookAbstract
New treatment strategies for acute myeloid leukemia (AML)/ acute lymphocytic leukemia (ALL)/chronic lymphocytic leukemia (CLL) patients are under development. Myeloid/Lymphoid leukemic cells (AML, ALL) can be differentiated into leukemia-derived dendritic cells (DCleu), potentially presenting the entire leukemic antigen repertoire without knowledge of distinct leukemia antigens, and are regarded as a promising candidate for a vaccination strategy. We investigated the effectiveness of three DC/DCleu-generating ‘Kits’, containing Interleukin-4 (IL-4) and CD40 Ligand (CD40L) (termed “Kit-1” ), containing Granulocyte-Macrophage-Colony-Stimulating-Factor (GM-CSF), Interleukin-4 (IL-4) and Tumor Necrosis Factor Alpha (TNFα) (termed “Kit-2” ), containing Granulocyte-Macrophage-colony-Stimulating-factor (GM-CSF) and Prostaglandin-E1 (PGE-1) (termed “Kit-M”), to enhance anti-leukemic effector/memory immune cells after T-cell-enriched mixed lymphocyte cultures (MLC), as induced by DC/DCleu generated ex vivo from AML, ALL, CLL patients` and Healthy donors` whole blood (WB). Kit-M (vs. Kit-1 and Kit-2) induced the strongest antileukemic effects in AML patients` WB. Mature DC/DCs were most effectively generated using Kit-1 (vs. Kit-2 and Kit-M) without inducing blast proliferation in ALL and CLL patients' WB and increased leukemia-specific immune and memory cells after MLC, leading to improved blast lysis. Overall, these findings underscore the potential of Kit-(especially Kit-M) induced DC/DCleu to evoke robust antileukemic immune responses and immunological memory against AML/ALL.
Keywords
<p style="text-align:justify">Acute lymphocytic leukemia (ALL); Acute myeloid leukemia (AML); Chronic lymphocytic leukemia (CLL); Anti-leukemic functionality; Leukemia-derived dendritic cells; Dendritic cell-based therapy; Immune monitoring.</p>
Article Details
Introduction
Leukemia
Acute myeloid (AML), lymphocytic (ALL), or chronic lymphocytic leukemia (CLL) are clonal diseases that arise from a malignant stem cell. Standard treatment for AML and ALL patients with advanced stages utilizes high-dose chemotherapy, followed by other (immune) treatments like stem cell transplantation (SCT) [1–3]. First-line treatment for CLL patients contains either a covalent Bruton tyrosine kinase (BTK) inhibitor or a B-cell leukemia/lymphoma 2 (BCL2) inhibitor [4]. But the rate of early failures and relapses, especially for AML or ALL, is still unsatisfying. Relapses could be avoided by inducing immune cells, specialized to fight residual leukemic blasts.
Innate and adaptive immune system and antileukemic process
Effective immune surveillance in patients with hematologic malignancies, such as leukemia, involves the coordinated activity of the innate and adaptive immune systems. The innate immune system consists of cells like macrophages, dendritic cells (DCs), and natural killer (NK) cells [5,6]. Leukemic blasts can be differentiated into leukemia-derived dendritic cells (DCleu), effectively presenting patient-specific leukemic antigens to immune cells. NK cells eliminate infected/tumor cells without prior activation, particularly those with downregulated major histocompatibility complex (MHC) class I molecules. NK cells exert their cytotoxic effects through the release of cytotoxic granules and death receptor-mediated apoptosis pathways, such as Fas ligand pathways. Cytokine-induced killer (CIK) cells, bridging innate and adaptive immunity, exhibit potent cytotoxic activity and can proliferate in the tumor microenvironment [7,8].
The adaptive immune system, which includes T and B cells, plays a critical role in antigen-specific tumor immunity and long-term immune memory. Naïve T cells are transformed into non-naïve T cells or memory cells (central or effector) memory cells, allowing faster immune reactivation against recurring antigens [9]. Regulatory T cells (Tregs), which suppress immune responses to maintain tolerance, can hinder anti-tumor immunity and enable tumor escape mechanisms. Adaptive immune cells like T Helper (e.g., Th1) and antileukemic specific T cells, as well as NK cells, produce cytokines like interferon-gamma (IFNγ) or start degranulation, thereby enhancing antigen-specific anti-tumor responses by activating other immune cells and directly inhibiting tumor growth [10,11]. The Intracellular Cytokine (INTCYT), Degranulation (DEG), and the Cytotoxicity Fluorolysis Assay (CTX) offer robust tools for assessing immune cell activity, functionality, and cytotoxicity against leukemia [12,13]. These immunoreactive cells can be detected using flow cytometry (abbreviations for cell subsets are given in Table 1).
Immunotherapy for AML, ALL and CLL
Immunotherapies using checkpoint or CD19/CD20 addressing mono/bispecific monoclonal antibodies or chimeric antigen receptor T-cell (CAR-T) therapy have become promising treatments improving survival for ALL or CLL patients [4,14]. However, immunotherapy for AML is currently less advanced due to the lack of highly specific surface markers on AML blasts. Monoclonal antibody-drug conjugate targeting CD33 archive cells (Ozogamicin) has shown efficacy in AML [15]. Different strategies have been developed with the attempt to redirect the immune system in order to overcome the leukemic immune escape and enforce a tumor-specific immune response. The integration of immunotherapy into treatment protocols is crucial to improve outcomes across these leukemia types [16–18].
DC-Based Immunotherapy as a Treatment Option
Dendritic cell (DC)-based immunotherapies have shown clinical efficacy [10,19,20]. Ex vivo DCs can be generated from CD14+ monocytes or from lymphoid/myeloid leukemic blasts (DCleu, leukemia-derived DC in WB samples of AML or ALL patients in the presence of different combinations of response modifier combinations (e.g., ranulocyte-macrophage-colony-stimulating-factor, GM-CSF; prostaglandin-E1, PGE1; Interleukin-4, IL-4; CD40 Ligand, CD40L and Tumor Necrosis Factor Alpha, TNFα).[5,21–35]. Kit-M (containing granulocyte-macrophage-colony-stimulating-factor, GM-CSF and prostaglandin-E1, PGE1) induced leukemia-derived DCs (DCleu) are characterized by the expression of the whole individual leukemic antigen repertoire [28,36,37] and have been shown to induce antileukemic reaction ex vivo after MLC (e.g., Klauer et al., 2025 [38] and Schutti et al.,2024 [11]). DCleu (presenting both DC- and individual patients’ blast antigens) generated by Kit-M have been shown to initiate (patient-specific) anti-leukemic immune responses in AML patients [39, 57].
Aim of the Study
This study aimed to evaluate:(1) The potential of Kit-1, Kit-2, and Kit-M to generate DCs and DCleu ex vivo from AML-, ALL-, CLL patients' and Healthy donors' whole blood (WB), thereby simulating in vivo conditions. (2) The potential of Kit-pretreated WB to induce antileukemic reactions after T-cell-enriched mixed lymphocyte culture. (3) And to deduce optimized protocols for the generation of DC/DCleu which reliably activate immunoreactive cells (eg, Tnaive, Tnon-naive, Tem/eff, NK, CIK) and improve antileukemic reactions ex vivo or potentially in vivo in patients with lymphoid or myeloid leukemia.
Table 1. Cells and cell subsets as evaluated by flow cytometry
Table 2. Patients’ characteristics
|
Patient No |
Age, Sex |
Stage |
WHO Classification |
Risk-Stratification (ELN/GMALL/NCCN) |
Blast phenotype (CD) |
% Blasts in WB |
Experiments Conducted with WB |
|
1419 |
65, f |
dgn. |
pAML (Ly) |
Adverse |
19, 34, 117, 13,15 |
94 |
DC, MLC, CTX |
|
1461 |
78, m |
dgn. |
pAML (Ly) |
Adverse |
19, 34, 22, 15, 65, 33 |
61 |
DC, MLC, CTX |
|
1568 |
29, m |
dgn. |
pAML (Ly) |
Intermediate |
19, 34, 20, 117, 13 |
60 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1574 |
56, m |
dgn. |
sAML (Ly) |
n.d. |
19, 34, 117, 15 |
33 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1565 |
62, f |
dgn. |
sAML (Myo) |
Intermediate |
34, 117, 13, 14 |
34 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1567 |
98, f |
dgn. |
pAML (Myo) |
Adverse |
34, 117, 15, 65 |
16 |
DC, MLC, Deg(UC), InCyt(UC), CTX |
|
1570 |
37, f |
dgn. |
pAML (Myo) |
Favourable |
34, 117, 65,13,33 |
8 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1572 |
63, f |
dgn. |
pAML (Myo) |
Adverse |
34, 117, 65, 33, 13 |
12 |
DC, MLC, Deg(C), InCyt(C), CTX |
|
1573 |
61, m |
dgn |
pAML (Myo) |
Adverse |
34, 117, 65, 13 |
13 |
DC, MLC, Deg(UC,C), InCyt(C), CTX |
|
1594 |
70, f |
dgn. |
pAML (Myo) |
Favorable |
34, 117, 65, 33, 56 |
2 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1641 |
65, f |
Rel.a.SCT |
tAML (Myo) |
n.d. |
34, 117, 33, 65 |
6 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1463 |
60, f |
Rel.a.SCT |
sAML (Ly) |
n.d. |
19, 34, 2, 3, 13, 56 |
6 |
DC, MLC, CTX |
|
1587 |
57, m |
dgn |
cALL (BII) |
High risk |
19, 34, 10, 15 |
33 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1605 |
56, f |
dgn. |
cALL(BII) |
High risk |
19, 34, 10, 20, 22 |
30 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1626 |
26, m |
dgn. |
cALL(BII) |
Standard |
19, 34, 10, 20, 22 |
20 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1646 |
26, m |
dgn. |
cALL(BII) |
High risk |
19, 34, 10, 20, 22 |
40 |
DC, MLC, Deg(UC,C), InCyt(UC,C) |
|
1653 |
77, m |
dgn. |
pro-B-ALL(BI) |
High risk |
19, 34, 22 |
55 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1662 |
46, m |
dgn. |
cALL(BII) |
Standard |
19, 10, 22, 65 |
48 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1670 |
23, m |
dgn. |
pro-B-ALL(BI) |
Standard |
19, 34, 3, 33 |
20 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1676 |
55, f |
dgn. |
cALL(BII) |
High risk |
19, 34, 10, 79a |
61 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1677 |
73, f |
dgn. |
pro-B-ALL(BI) |
Standard |
19, 34, 22, 65, 79a |
26 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1588 |
61, m |
Rel. |
cALL (BII) |
Standard |
19, 34, 10, 20, 23 |
2 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1657 |
35, m |
Rel. |
Pre-T-ALL (BII) |
High risk |
5, 34, 2, 4, 7 |
30 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1589 |
61, f |
Pers. |
B-CLL |
A |
19, 20, 5, 15, 23 |
45 |
DC, MLC, CTX |
|
1591 |
82, m |
Pers. |
B-CLL |
A |
19, 5, 15, 20 |
52 |
DC, MLC, CTX |
|
1639 |
75, f |
Pers. |
B-CLL |
n.d. |
19, 22, 23 |
57 |
DC, MLC, Deg(UC,C), InCyt(UC,C),CTX |
|
1678 |
50, m |
Pers. |
B-CLL |
A |
19, 5, 20, 22, 23, 79b |
80 |
DC, MLC, Deg(UC,C), InCyt(UC,C), CTX |
|
1681 |
66, m |
Pers. |
B-CLL |
A |
19, 5 |
83 |
DC, MLC, Deg(C), InCyt(C), CTX |
|
1687 |
37, m |
Pers. |
B-CLL |
A |
19, 5, 20 |
17 |
DC, MLC, Deg(UC), InCyt(UC) |
|
1688 |
66, m |
Pers. |
B-CLL |
A |
19, 5, 20, 79b |
65 |
DC, MLC, Deg(UC), InCyt(UC) |
|
1563 |
26, f |
Healthy |
DC, MLC |
||||
|
1566 |
54, f |
Healthy |
DC, MLC, Deg(UC), InCyt(UC) |
||||
|
1652 |
43, m |
Healthy |
DC, MLC |
||||
|
1661 |
34, f |
Healthy |
DC, MLC, Deg(UC,C), InCyt(UC,C) |
||||
|
1666 |
38, m |
Healthy |
DC, MLC, Deg(UC,C), InCyt(UC,C) |
||||
|
1667 |
25, f |
Healthy |
DC, MLC, Deg(UC,C), InCyt(UC,C) |
||||
|
1668 |
60, f |
Healthy |
DC, MLC, Deg(UC,C), InCyt(UC,C) |
Legend: f: female; m: male; WHO classification: World Health Organization classification: acute myeloid leukemia; pAML: preliminary AML; sAML secondary AML; tAML: therapy-related AML; AML(Ly): AML blasts with lymphoid marker expression; AML(Myo): AML blasts without lymphoid marker expression; ELN: European Leukemia Network; NCCN: National Comprehensive Cancer Network; GMALL: German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia; dgn.: first diagnosis; Pers.: persistence; Rel.: relapse; Rel. a. SCT: relapse after SCT; WB: whole blood; CD: Cluster of differentiation; bold: antibody used for expression analyses; n.d.: no data; DC: dendritic cell culture; MLC: mixed lymphocyte culture; Deg: degranulation assay; InCyt: intracellular cytokine assay; CTX: cytotoxicity assay; UC: measurements in uncultured samples; C: measurements after culture.
Materials and Methods
Sample Collection
Sample acquisition was conducted after obtaining informed consent of the blood donor and in accordance with the Helsinki protocol and the ethics committee of the Ludwig-Maximilians-University-Hospital Munich (Vote-No 339-05). Heparinised peripheral whole blood (WB) samples were provided by the University Hospitals of Munich, Augsburg, Oldenburg, Tübingen, Frankfurt, the Rotkreuzklinikum in Munich, the Diakonieklinikum in Stuttgart, and the St.-Josefs-Hospital in Hagen between 2016 and 2023. More details on patients’ characteristics can be found in the supplementary material.
Flow Cytometry to characterize and quantify cell subsets and their functionality
To evaluate and quantify phenotypes of DC/DCleu, leukemic blasts, monocytes, and immune-reactive cell subsets of the adaptive and innate immunity, analyses were conducted via flow cytometry, using a fluorescence-activating cell-sorting flow cytometer (FACS CaliburTM). Using a refined gating technique and the analysis software Cell Quest-Pro (Becton Dickinson, Heidelberg, Germany), functionalities of cells (proliferation, cytokine production, degranulation, and cytotoxicity) could be investigated, as shown before [12]. Evaluation and quantification of stained cells was obtained with the fluorescence-activated cell sorting flow cytometer FACS Calibur (Becton Dickinson) and the analysis software Cell Quest-Pro 6.1 (Becton Dickinson), applying a refined gating strategy, as shown before [35]. For more details, refer to the supplementary material.
Dendritic Cell Culture (DCC)
The generation of DC/DCleu from healthy and leukemic WB was performed using 3 different DC-generating methods: “Kit-1” containing 20ug/mL IL-4 (ThermoFischer Scientific, Darmstadt, Germany), 3ug/mL CD40L(ThermoFischer Scientific, Darmstadt, Germany); “Kit-2” containing 800U/ml GM-CSF; Sanofi-Aventis, Frankfurt, Germany), 20ug/mL IL-4 (ThermoFischer Scientific, Darmstadt, Germany) and 10ng/mL Tumor Necrosis Factor Alpha (TNFα; ThermoFischer Scientific, Darmstadt, Germany) and “Kit-M” containing 800 U/mL GM-CSF; Sanofi-Aventis, Frankfurt, Germany) and 1 μg/mL Prostaglandin-E1 (PGE1; Santa Cruz Biotechnology) [21–35]. For more details, refer to the supplementary material.
T Cell-Enriched Mixed Lymphocyte Culture (MLC)
To generate T cell-enriched immune-reactive cells, thawed autologous T cells were stimulated with DC/DCleu containing Kit-pretreated WB. Flow cytometric analyses of T-cell subsets were quantified using a refined gating strategy, as shown before [35]. For more details, refer to the supplementary material.
Intracellular Cytokine Assay (INTCYT) and Degranulation Assay (DEG)
INTCYT and DEG cultures were set up as described [35] to detect intracellular IFNγ producing leukemia-specific cells and leukemia-specific cells, as given in Table 1. Flow cytometric analyses of the INTCYT- and the DEG-assays were quantified using a refined gating strategy. For more details, refer to the supplementary material.
Cytotoxicity Fluorolysis Assay (CTX)
The Cytotoxicity Fluorolysis Assay was performed to analyse the blast lytic activity of T cell-enriched immune-reactive cells in MLCWB-DC(Kit-1, Kit-2, Kit-M) and MLCWB-DC(Control). Flow cytometric analyses were performed using a refined gating strategy. Achieved blast lytic activity was defined as the percentage difference of viable target cells (blasts) between the effector-target-cell culture and the control, as shown before [35]. For more details, refer to the supplementary material.
Statistical Methods
Statistical analyses and figures were implemented with Excel 2022 (Microsoft, Redmond, WA, USA) and Prism 9 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard deviation. In some comparisons/correlations, relatively changed frequencies of cell subsets (deltas) between Kit-treated and untreated settings are given. Statistical analyses were conducted using t-tests: Differences were considered as “highly significant” in cases with p-values ≤ 0.005, as “significant” with p-values ≤ 0.05, and as “borderline significant” with p-values between 0.05 and 0.10.
Results
Prologue
In our samples, between 2% and 94% of blasts and varying proportions of the remaining hematopoietic cells were detectable. Details of the cellular composition of AML/ ALL /CLL samples are shown in Table 2. In our study, we analyzed uncultured immune reactive cells from AML, ALL, CLL, and healthy donors´ WB samples and samples after the influence of several Kits for DC generation. In two patients with low blasts (<5%), DC/DCleu could not be quantified. Furthermore, we quantified (leukemia-specific) immune cells (IFNγ-producing/degranulating T, NK, CIK, B-cells) following DC/MLC culture. We correlated results with (ex vivo) functional as well as patients’ clinical and prognostic data.
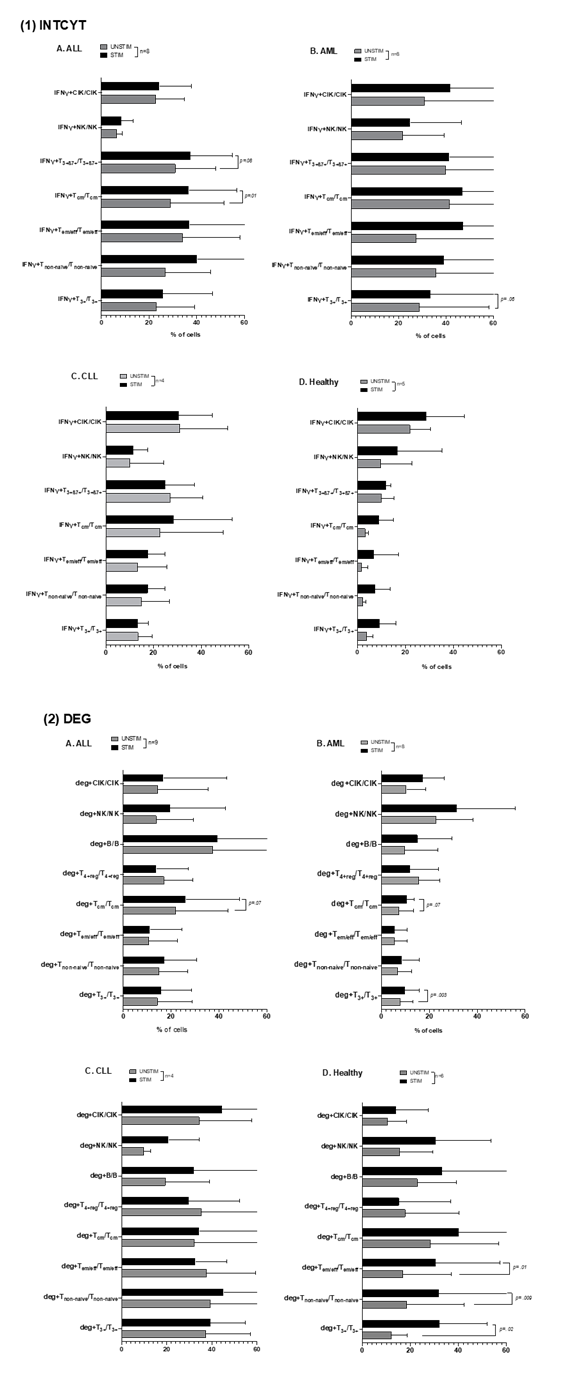
Stimulation of uncultured (AML, ALL, CLL, and Healthy) WB with LAA/SEB increases frequencies of (antigen-specific) intracellularly IFNγ–producing and degranulating immune cells
In AML patients´ uncultured WB, frequencies of leukemia-specific intracellularly IFNγ-producing or degranulating cells were comparable (Fig.1.B), thereby confirming data already demonstrated before (e.g., Unterfrauner et al., 2023 [37]). The frequencies of intracellular IFNγ-producing and degranulating adaptive and innate immune cells (e.g, Tnon-naive, Tcm, Tem/eff, CIK, NK cells) are increased (or decreased: T4+reg) after the addition of leukemic (WT1, PRAME) antigens. The results of AML-Myo and AML-Ly were comparable (data not shown). In ALL patients´ uncultured WB, we found non-significantly higher frequencies of intracellular IFNγ-producing cells with LAA (STIM) vs. without LAA stimulation (UNSTIM) (e.g., IFNγ+Tcm/Tcm, IFNγ+T3+ß7+/T3+ß7+). We observed significantly or borderline higher frequencies (e.g., % IFNγ+Tcm/Tcm: UNSTIM: 28.93±20.88, STIM: 36.72±18.56, p= 0.01; IFNγ+T3+ß7+/T3+ß7+: UNSTIM: 31.05±15.93, STIM: 37.49±16.30, p= 0.08) compared to unstimulated WB (Fig.1.A(1)). For degranulating T cells or B cells with/without LAA stimulation, differences were pronounced (Fig.1.A(2)). In CLL patients´ uncultured WB, we observed no significant differences in intracellular IFNγ-producing or in degranulating cells with vs. without LAA stimulation (Fig.1.C). In healthy samples exposed to Staphylococcal Enterotoxin B (SEB) stimulation, we observed (non)significantly higher frequencies of intracellular IFNγ-producing and degranulating cells with vs. without SEB stimulation (Fig.1.D), thereby confirming data shown before [11].
Mean frequencies ± standard deviation of uncultured immune cells producing intracellularly IFNγ (IFNγ+) (Fig.1.(1)) or expressing CD107a (deg+)(Fig.1.(2)) in leukemic patients’ WB (ALL(A), AML(B), CLL(C)) and healthy donors’ WB(D) with vs. without LAA (leukemic samples) or SEB (healthy) stimulation (STIM) are provided; Statistical analyses were conducted using t-test: Differences were considered as highly significantly different with p values ≤ 0.005, as significantly different with p values ≤0.05 and as borderline significantly different with p values between 0.05 to 0.10. Abbreviations of cell types are given in Table 1.
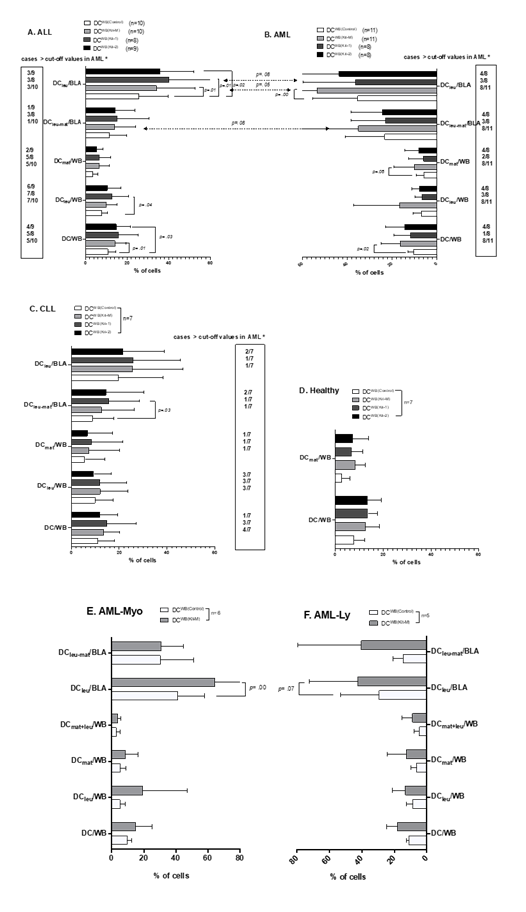
(Leukemia) derived DC/DCleu are increased in AML, ALL, CLL patients, or healthy donors’ WB, depending on the influence of Kit-1, Kit-2, and Kit-M compared to Control
In AML patients´ samples, we confirm data already demonstrated before (e.g., Unterfrauner et al., 2023 [37]), that the frequencies of (mature/leukemia-derived) DC can be significantly increased under the influence of Kit-M (vs control) without induction of AML blasts’ proliferation (data not shown). Additionally, we found only non-significantly higher frequencies of DC/DCleu in Kit-1 and Kit-2 pretreated WB compared to the control. In a detailed analysis, we confirm that more cases with higher DC/DCleu values compared to control were found in AML using Kit-M compared to Kit-1 or Kit-2-pretreatment (e.g., DC/WB: Kit-1: 1/8 (1 of 8 cases); Kit-2: 4/8; Kit-M: 8/11; DCleu/BLA: Kit-1: 3/8; Kit-2: 4/8; Kit-M: 8/11) (Fig.2.B). The results of DC/DCleu in AML-Myo and in AML-Ly´ WB under the influence of Kit-M were comparable (Fig.2.E, F) (in one case, with <5% blasts in PB, DC could not be quantified). In ALL patients´ samples, we generated higher average frequencies of leukemia derived DC/DCleu subtypes following treatment with Kit-1 (DCWB (Kit-1)), Kit-2 (DCWB (Kit-2)) and Kit-M (DCWB (Kit-M)) compared to control group (DCWB(Control)) (e.g., %DC/WB: Kit-1:13.59±7.85; Kit-2: 12.27±3.58; Kit-M: 13.33±4.08; Control: 9.37±2.18; p= 0.12, p= 0.003, p= 0.008; %DCleu/BLA: Kit-1: 35.59±20.39; Kit-2: 33.83±13.7; Kit-M: 32.28±16.54; Control: 22.60±9.32; p= 0.01, p= 0.02; p= 0.01) (in one case with <5% blasts in PB DCleu could not be quantified). Proliferation of blasts (as detected by the co-expression of CD71 or IPO-38) from leukemic WB was not induced by Kit-M, Kit-1, and Kit-2 treatment (data not shown).
In summary, we observed higher frequencies of DCs and their subtypes in Kit-1-treated ALL-WB, followed by Kit-M and Kit-2, compared to the control group. Compared to AML, in general frequencies of DC/DCleu subtypes were (non-significantly) lower. In a detailed analysis, we defined “cut-off” values with respect to frequencies of DC subtypes obtained in > two-thirds of AML cases using Kit-M. In ALL cases, more cases with DC/DCleu were seen using Kit-1 followed by Kit-2 and Kit-M-pretreatment (e.g., DC/WB: Kit-1: 5/8 (5 of 8 cases); Kit-2: 4/9; Kit-M: 5/10; DCleu/BLA: Kit-1: 3/8; Kit-2: 3/9; Kit-M: 3/10) (Fig.2.A).
Comparing the results between ALL and AML patients´ samples, we found that AML samples, treated with Kit-M, generated more DCleu/BLA (p= 0.05) and DCleu-mat/BLA (p= 0.08) (Fig.2.A and B). In CLL patients´ samples, we found slightly increased frequencies of DC/DCleu/DCleu-mat in the blasts´ fractions using Kit-M or Kit-1 vs. Control (e.g., %DCleu-mat/BLA: Kit-1: 15.61±11.89; Control: 12.64 ±12.64, p= 0.03). Proliferation of blasts was not induced by Kit-M, Kit-1, and Kit-2 treatment (data not shown). Compared to ALL and AML patients´ samples, fewer cases with DC/DCleu values higher than the control values were found (Fig.2.C). Due to the low number of CLL cases, a comparison with AML patients´ samples was not performed. In healthy samples, we found non-significantly higher frequencies of DC within WB when using Kit-M (DCWB (Kit-M)), Kit-1 (DCWB (Kit-1)), Kit-2 (DCWB (Kit-2)) compared to control (DCWB(Control)) (Fig.2.D).
Mean frequencies ± standard deviation of generated DC subtypes in leukemic patients’ WB (Fig.2. A, B, C, E, F) and generated DC subtypes and monocytes in healthy donors’ WB (Fig.2.D) (with and without Kit-1, Kit-2, or Kit-M pretreatment of WB (DCWB(Control); DCWB (Kit-M), DCWB (Kit-1), DCWB (Kit-2)). AML-Myo cases expressing myeloid antigens, AML-Ly cases expressing additionally lymphoid antigens. Statistical analyses were conducted using a t-test: Differences were considered as highly significantly different with p values ≤ 0.005, as significantly different with p values ≤0.05, and as borderline significantly different with p values between 0.05 to 0.10. Abbreviations of cell types are given in Table 1.
*″Cut-off ″ Cut-off values defined with respect to frequencies of WB or Blasts in > two-thirds of AML cases after using Kit-M treatment (box).
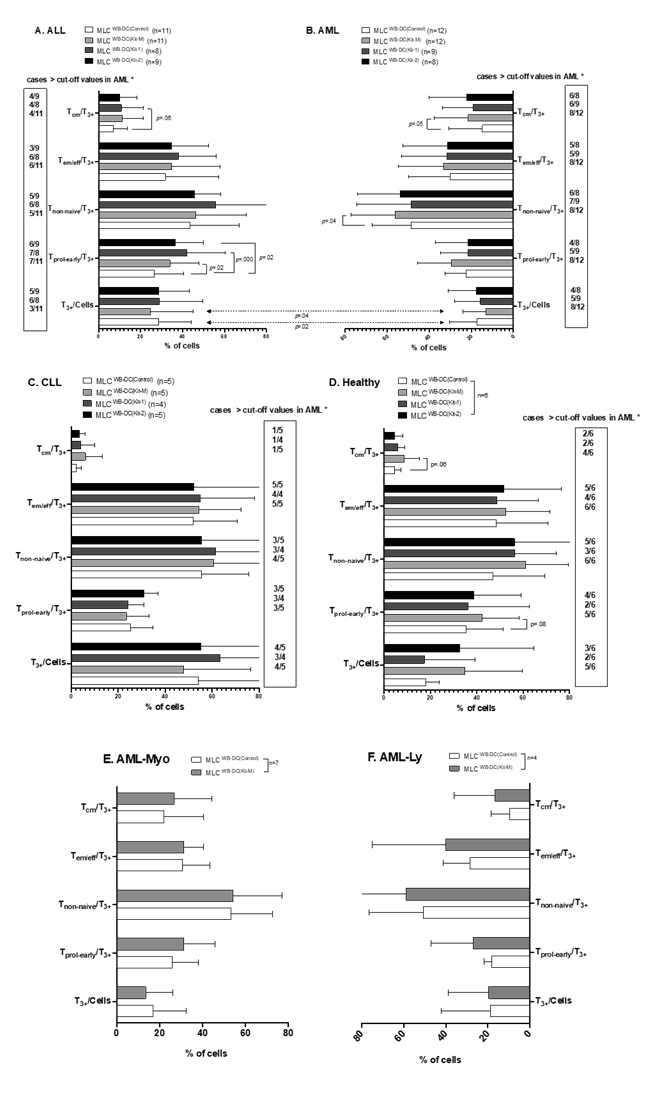
Activated and memory T cells are increased after MLC with Kit-1, Kit-2, and Kit-M- pretreatment of patients’ or healthy donors’ WB
In AML patients´ samples, we found significantly higher frequencies of Tcm subsets and non-naïve T cell subsets in MLCWB-DC(Kit-M) compared to MLCWB-DC(Control) (%Tcm/T3+: Kit-M: 22.53±15.55; Control: 14.92±15.23; p= 0.05 and %Tnon-naive/T3+: Kit-M: 55.96±19.95; Control: 48.63±17.64; p= 0.04). In a detailed analysis, we confirmed that there were more cases with higher frequencies of Tnon-naïve and Tcm in AML using Kit-M than Kit-1 or Kit-2-pretreatment (e.g., Tprol-early/T3+: Kit-1: 5/9; Kit-2: 4/8; Kit-M: 8/12; Tem/eff/T3+: Kit-1: 5/9 (5 of 9 cases); Kit-2: 5/8; Kit-M: 8/12) (Fig.3.B). Comparable results were obtained comparing AML-Myo and AML-Ly of T cell subsets after MLC with Kit-M-pretreated WB (MLCWB-DC(Kit-M) (Fig.3.E, F).
In ALL patients´ samples, we found (significantly) higher frequencies of early proliferating T cells, Tnon-naïve or Tcm in MLCWB-DC(Kit-1), MLC WB-DC(Kit-2), and MLCWB-DC(Kit-M) compared to MLCWB-DC(Control) (e.g.: %Tprol-early/CD3+: Kit-1: 42.06±17.17, Kit-2: 36.64±12.42, Kit-M: 34.08±13.07, Control: 26.53±13.19; p= 0.000, p= 0.02, p= 0.02), with the highest values obtained with Kit-1. Moreover, we defined “cut-off” values with respect to frequencies of cell subsets obtained in > two-thirds of AML cases using Kit-M. We found that, in ALL using Kit-1-pretreatment, more cases with higher activated and memory T cells than with Kit-2- or Kit-M-pretreatment were found (e.g., Tnon-naive/T3+: Kit-1: 6/8; Kit-2: 5/9; Kit-M: 5/11; Tcm/T3+: Kit-1: 4/8 (4 of 8 cases); Kit-2: 4/9; Kit-M: 4/11) (Fig.3.A). In CLL patients´ samples, we did not find higher frequencies of T cell subsets after MLC with Kit-1, Kit-2, and Kit-M pretreated WB compared to control (Fig.3.C). In healthy samples, we found borderline significantly higher frequencies of Tcm and Tprol-early in MLCWB-DC(Kit-M) compared to MLCWB-DC(Control) (%Tcm/ T3+: Kit-M: 8.62±6.16; Control: 4.46±2.55; p= 0.06 and %Tprol-early/T3+: Kit-M: 42.29±14.57; Control: 35.37±14.74; p= 0.08). We also found (non-significantly) increased frequencies of Tnon-naïve/T3+ in MLCWB-DC(Kit-1) and MLCWB-DC(Kit-2) compared to MLCWB-DC(Control) (Fig.3.D).
Mean frequencies ± standard deviation of T cell subsets after stimulation of T cell-enriched immunoreactive cells containing Kit-1, Kit-2, or Kit-M pretreated WB (MLC WB-DC(Kit-1), MLC WB-DC(Kit-2), MLC WB-DC(Kit-M)) from leukemic (Fig.3. A, B, C, E, F) or healthy (Fig.3.D) T cells. Statistical analyses were conducted using a t-test: Differences were considered as highly significantly different with p values ≤ 0.005, as significantly different with p values ≤0.05, and as borderline significantly different with p values between 0.05 to 0.10. Abbreviations of cell types are given in Table 1.
*″Cut-off ″ Cut-off values defined with respect to frequencies of T cell subsets in > two-thirds of AML cases after using Kit-M treatment (box).
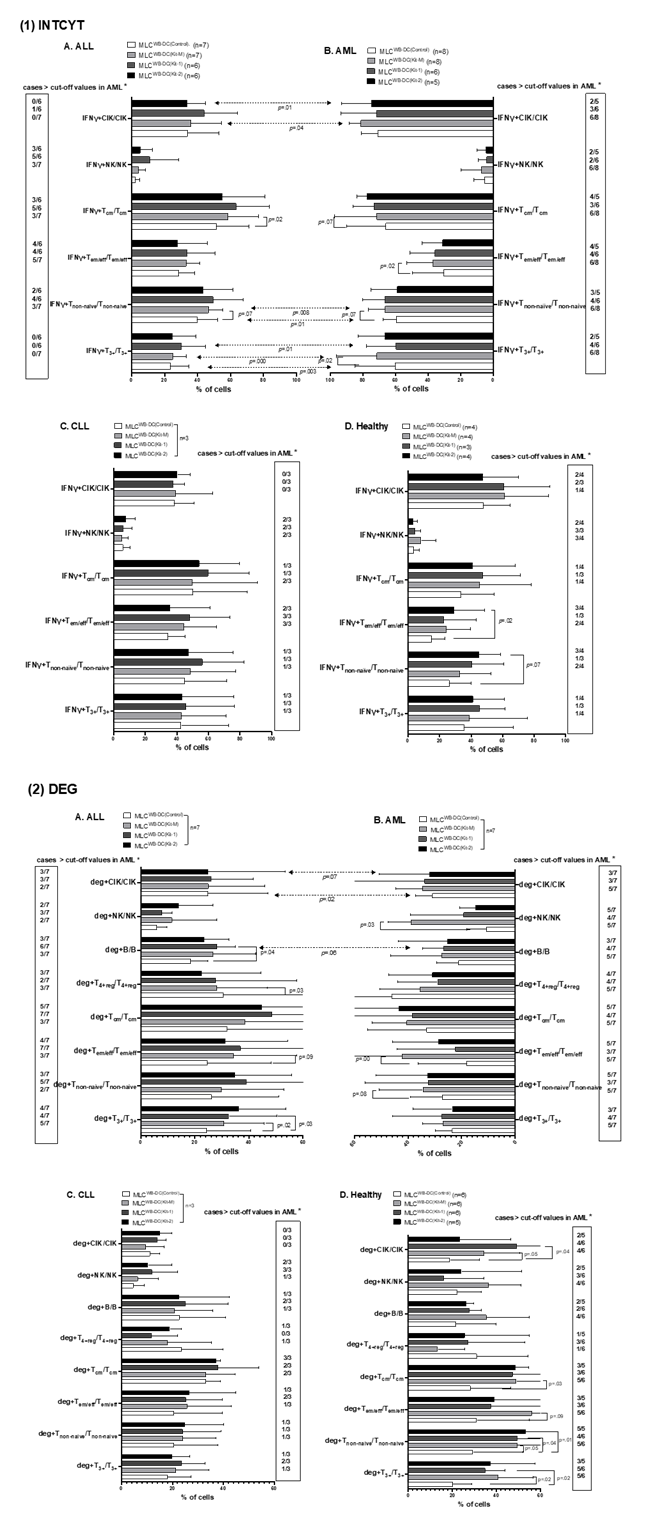
Increased frequencies of intracellular IFNγ-producing and degranulating immune cells after MLC, depending on Kit-1, Kit-2, and Kit-M-pretreatment of AML, ALL, CLL, and healthy donors´ WB
In AML, compared to Kit-1 and Kit-2, frequencies of intracellular IFNγ+ producing or degranulating cells were significantly upregulated in samples pretreated (vs not pretreated) with Kit-M-pretreatment (e.g., % deg+Tem/eff/ Tem/eff: MLC WB-DC(Kit-M): 37.16±14.23%, MLC WB-DC(Control): 30.53±17.08%, p= 0.00; deg+NK/NK: MLC WB-DC(Kit-M): 7.27±11.37%, MLC WB-DC(Control): 5.51±5.72%, p= 0.03). Moreover, we also confirmed that there were more cases with increased intracellular IFNγ-producing and degranulating immune cells after MLC in AML using Kit-M than Kit-1-pretreatment (e.g., IFNγ+Tcm+/Tcm: Kit-1: 3/6 (3 of 6 cases); Kit-2: 4/5; Kit-M: 6/8; deg+Tnon-naive/Tnon-naive: Kit-1: 3/7; Kit-2: 5/7; Kit-M: 5/7; deg+Tcm/Tcm: Kit-1: 3/7; Kit-2: 5/7; Kit-M: 5/7) (Fig.4.B). Frequencies of intracellular IFNγ+ producing or degranulating cells of AML-Myo and AML-Ly were comparable. However, due to the low number of cases, studied data are not presented in detail.
In ALL patients´ samples, we observed (non-significantly) increased intracellular IFNγ-producing immune cells using Kit-pretreatment after MLC compared to control (e.g., %IFNγ+Tcm/Tcm: MLC WB-DC(Kit-1): 63.36±16.44, MLC WB-DC(Kit-2): 55.04±23.04, MLC WB-DC(Kit-M): 58.42±17.18, MLC WB-DC(control): 51.44±18.15; p= 0.24, p= 0.34, p= 0.07) (Fig.4.A(1)). Additionally, we found significantly higher frequencies of deg+T3+/T3+ and deg+B/B in MLC WB-DC(Kit-1) compared to MLC WB-DC(Control). (e.g., % deg+T3+/T3+: MLC WB-DC(Kit-1): 32.51±16.49, MLC WB-DC(Control): 24.43±14.92, p= 0.03; deg+B/B: MLC WB-DC(Kit-1): 28.07±6.25, MLC WB-DC(Control): 18.54±5.67, p= 0.04). We demonstrated a significant decrease in frequencies of deg+T4+reg/T4+reg in Kit-M treated samples compared to the control group (Fig.4.A(2)). Moreover, we defined “cut-off” values with respect to cell frequencies obtained in > two-thirds of AML cases using Kit-M. We found that, in ALL using Kit-1-pretreatment, there were more cases with increased intracellularly IFNγ producing and degranulating immune cells after MLC than with Kit-2 or Kit-M-pretreatment (e.g., IFNγ+Tnon-naive/Tnon-naive: Kit-1: 4/6 (4 of 6 cases); Kit-2: 2/6; Kit-M: 3/7; IFNγ+Tcm+/Tcm: Kit-1: 5/6; Kit-2: 3/6; Kit-M: 3/7; deg+Tnon-naive/Tnon-naive:Kit-1: 5/7; Kit-2: 3/7; Kit-M: 2/7; deg+Tcm/Tcm: Kit-1: 7/7; Kit-2: 5/7; Kit-M: 3/7) (Fig.4.A). We conclude that Kit-1-pretreatment, followed by Kit-M (vs. no treatment), led to the most effective increase in production of intracellular IFNγ and degranulation activity in ALL samples’ adaptive immune cells.
In three CLL samples, we found (non-significantly) upregulated frequencies of intracellular IFNγ+ producing or degranulating cells in samples with Kit-1-pretreatment (vs. no treatment) (Fig.4.C). In healthy samples, we found significantly higher frequencies of antigen-specific deg+T3+/T3+, deg+Tnon-naive/Tnon-naive, deg+Tcm/Tcm, deg+CIK/CIK, comparing MLC WB-DC(Kit-M) with MLC WB-DC(Control). (e.g., % deg+Tcm/Tcm: MLC WB-DC(Kit-M): 49.00±22.78%, MLC WB-DC(Control): 28.26±16.55%, p= 0.03). And we also observed (non-significantly) decreased frequencies of deg+T4+reg compared to MLC WB-DC(Kit-M), MLC WB-DC(Kit-1), and MLC WB-DC(Kit-2) with MLC WB-DC(Control) (Fig.4.D).
Mean frequencies ± standard deviation of Kit-1, Kit-2 or Kit-M-pretreated immune cells (MLCWB-DC(CTR), MLCWB-DC(KIT-1), MLCWB-DC(KIT-2), MLCWB-DC(KIT-M)) producing intracellular IFNγ (IFNγ+) (Fig.4.(1) or expressing CD107a (deg+) (Fig.4.(2)) in leukemic patients’ WB (A, B, C) and healthy donors’ WB (D) are provided; Statistical analyses were conducted using t-test: Differences were considered as highly significantly different with p values ≤ 0.005, as significantly different with p values ≤0.05 and as borderline significantly different with p values between 0.05 to 0.10. Abbreviations of cell types are given in Table 1.
*″Cut-off ″ Cut-off values defined with respect to frequencies of T cell subsets and B cells in > two-thirds of AML cases after using Kit-M treatment (box).
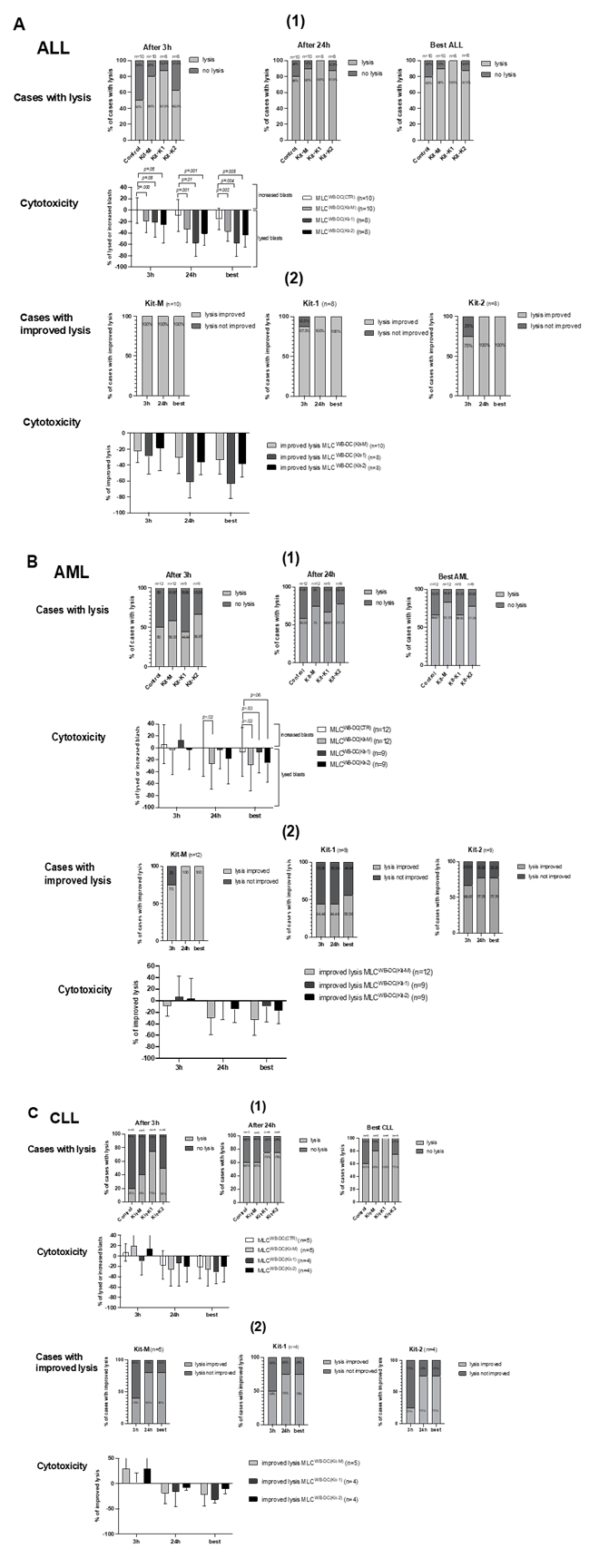
Increased antileukemic cytotoxicity after MLC of Kit-1, Kit-2, and Kit-M-pretreated (vs. untreated) AML, ALL, and CLL patients’ WB
After co-culture of ‘effector cells’ (T cell-enriched MLC with Kit-1/Kit-2/ Kit-M-pretreated (vs. not pretreated WB) with ‘target cells’ (thawed blast-containing MNC), we compared blast lysis of MLCWB-DC(Kit-1) (‘Kit-1’), MLCWB-DC(Kit-2) (‘Kit-2’), and MLCWB-DC(Kit-M) (‘Kit-M’) vs. MLCWB-DC(Control) (‘Control’), using a cytotoxicity fluorolysis assay. Analyses were conducted after 3 or 24 h of incubation of the target with effector cells, and finally, the best antileukemic effectivity after either 3 or 24 h was selected as the ‘best’ achieved lysis value. The lytic activity was calculated and defined as the frequency of (non-)viable target cells compared to a control [35,37].
- AML
After 3 hours of coincubation of effector with target cells, blast lysis was observed in 44.44% of cases (4/9) after Kit-1-pretreatment (MLCWB-DC (Kit-1)), 66.67% of cases (6/9) after Kit-2 pretreatment (MLCWB-DC(Kit-2)), and 58.33% of cases (7/12) after Kit-M-pretreatment (MLCWB-DC (Kit-M)) vs. 50% of cases (6/12) in the Control (MLCWB-DC(Control)). After 24 hours, blast lysis was observed in 66.67% of cases (6/9) after Kit 1-pretreatment (MLCWB-DC(Kit-1)), 77.76% of cases (7/9) after Kit-2 pretreatment (MLCWB-DC(Kit-2)), and 75% of cases (9/12) after Kit-M-pretreatment (MLCWB-DC (Kit-M)) vs. 58.33% of cases (7/12) in the Control (MLCWB-DC(Control)).
Selecting the ‘best’ achieved lysis value after 3 h and 24 h of incubation time, we found more cases with lysis after MLC WB-DC (Kit-M) vs. MLCWB-DC(Control) (MLCWB-DC(Kit-M): 83.33% vs. MLCWB-DC(Control): 56.67% cases with lysis). Frequencies of lysed blasts in MLCWB-DC(Kit-M), MLCWB-DC(Kit-2) were (borderline/significantly) lower compared to MLCWB-DC(Control) (%lysed blasts: MLCWB-DC(Kit-M): 29.16±41.13% vs. MLCWB-DC(Control): 6.9±38.79%; p= 0.02; MLCWB-DC(Kit-2): 25.41±30.93% vs. MLCWB-DC(Control): 6.9±38.79%, p= 0.06) (Fig.5.B(1)). After 24 hours of incubation of effector with target cells, all cases showed improved blast lysis after MLCWB-DC(Kit-M) compared to MLCWB-DC(Control), which led to improved lysis of 33.05±25.99% (Fig.5.B(2)). In summary, compared to the effects seen after Kit-1 and Kit-2-pretreatment, the most effective and improved blast killing was achieved after pretreatment vs. no pretreatment of AML samples with Kit-M, thereby confirming data shown before (e.g, Unterfrauner 2023 [37]).
- ALL
After 3 hours of coincubation of effector with target cells, blast lysis was observed in 87.5% of cases (7/8) after Kit-1-pretreated (MLCWB-DC (Kit-1)), 62.5% of cases (5/8) after Kit-2 pretreated (MLCWB-DC(Kit-2)), and 80% of cases (8/10) after Kit-M-pretreated (MLCWB-DC (Kit-M)), vs. 50% of cases (5/10) in the Control (MLCWB-DC(Control)). After 24 hours, blast lysis was observed in 100% of cases (8/8) after Kit-1-pretreated (MLCWB-DC(Kit-1)), 87.5% of cases (7/8) after Kit-2 pretreated (MLCWB-DC(Kit-2)), and 90% of cases (9/10) after Kit-M-pretreated (MLCWB-DC (Kit-M)), vs. 80% of cases (8/10) in the Control (MLCWB-DC(Control)). Selecting the ‘best’ achieved lysis value after 3 h and 24 h of incubation time, we found more cases with lysis after MLC WB-DC (Kit-1) vs. MLCWB-DC(Control) (MLCWB-DC(Kit-1): 100% vs. MLCWB-DC(Control): 80% cases with lysis. Frequencies of lysed blasts in MLCWB-DC(Kit-1), MLCWB-DC(Kit-2) and MLCWB-DC(Kit-M) were (borderline/significantly) lower compared to MLCWB-DC(Control) (%lysed blasts: MLCWB-DC(Kit-1): 58.19±21.60% vs. MLCWB-DC(Control): 15.57±18.08%, p= 0.004; MLCWB-DC(Kit-2): 43.68±20.24% vs. MLCWB-DC(Control): 15.57±18.08%, p= 0.005; MLCWB-DC (Kit-M): 37.34±16.75% vs. MLCWB-DC(Control): 15.57±18.08%, p= 0.002) (Fig.5.A(1)). We found cases with improved blast lysis in all cases (100%) after MLCWB-DC(Kit-1), MLCWB-DC(Kit-2), and MLCWB-DC(Kit-M) compared to MLCWB-DC(Control), which led to improved blast lysis of 63.36±17.43% in MLCWB-DC(Kit-1), 38.29±15.70% in MLCWB-DC(Kit-2), and 33.44±17.43% in MLCWB-DC(Kit-M) (Fig.5.A(2)). In summary, ALL patients’ samples pretreated with different Kits (Kit-1followed by Kit-2/Kit-M) achieved significantly improved blast lysis after MLC.
- CLL
After 3 hours of coincubation of effector with target cells, blast lysis was observed in 75% of cases (3/4) after Kit-1-pretreatment (MLCWB-DC (Kit-1)), 50% of cases (2/4) after Kit-2 pretreatment (MLCWB-DC(Kit-2)), and 40% of cases (2/5) after Kit-M-pretreatment (MLCWB-DC (Kit-M)) vs. 20% of cases (1/5) in the Control (MLCWB-DC(Control)). After 24 hours, blast lysis was observed in 75% of cases (3/4) after Kit-1-pretreatment (MLCWB-DC(Kit-1)), 75% of cases (3/4) after Kit-2-pretreatment (MLCWB-DC(Kit-2)), 60% of cases (3/5) after Kit-M-pretreatment (MLCWB-DC (Kit-M)) vs. 60% of cases (3/5) in the Control (MLCWB-DC(Control)). Selecting the ‘best’ achieved lysis value after 3 h and 24 h of incubation time, we found more cases with lysis after MLC WB-DC (Kit-1) vs. MLCWB-DC(Control) (MLCWB-DC(Kit-1): 100% vs. MLCWB-DC(Control): 60% cases of lysis. Frequencies of lysed blasts in MLCWB-DC(Kit-1), MLCWB-DC(Kit-2), MLCWB-DC(Kit-M) were (non-significantly) lower compared to MLCWB-DC(Control) (Fig.5.C(1)).We found improved blast lysis in 75% of cases (100%) after MLCWB-DC(Kit-1) and MLCWB-DC(Kit-2), in 80% of cases in MLCWB-DC(Kit-M) compared to MLCWB-DC(Control), which led to improved lysis of 31.91±5.51% in MLCWB-DC(Kit-1), 10.81±8.22 % in MLCWB-DC(Kit-2), and 22.23±19.79% in MLCWB-DC(Kit-M) (Fig.5.C(2)). In summary, CLL patients’ samples treated with different Kits (Kit-1followed Kit-2/Kit-M) showed less improved blast lysis after MLC compared to ALL or AML.
Stimulatory effect of Kit-1, Kit-2, or Kit-M-pretreated (vs. untreated) WB on the cytotoxic activity after 3 h and 24 h of co-culture of immunoreactivity cells (‘effector cells’) and blasts (‘target cells’) of ALL, AML, and CLL. Given are the proportions of cases with blast lysis and the frequencies ± standard deviation of increased or lysed blasts after MLCWB-DC(Kit-1) (Kit-1) MLCWB-DC(Kit-2) (Kit-2) and MLCWB-DC(Kit-M) (Kit-M) compared to MLCWB-DC(Control) (Control) after 3h and 24h and the ‘best’ achieved blast lysis after 3h or 24h (Fig.5(1)); Given are the proportions of cases with an improvement in blast lysis and the frequencies ± standard deviation of improved blast lysis after MLCWB-DC(Kit-1), MLCWB-DC(Kit-2) and MLCWB-DC(Kit-M) in relation to MLCWB-DC(Control) after 3h and 24h and the ‘best’ achieved improvement in blast lysis after 3h or 24h (Fig.5(2)). Statistical analyses were conducted using a t-test: Differences were considered as highly significantly different with p values ≤ 0.005, as significantly different with p values ≤0.05, and as borderline significantly different with p values between 0.05 to 0.10. Abbreviations of cell types are given in Table 1.
Discussion
Challenges in ALL, AML, and CLL treatment
Maintaining remission in the treatment of AML, ALL, and CLL remains a major clinical challenge, since blasts survive (initial) therapy. Leukemic cells evade immune attack through various escape mechanisms (e.g., modified HLA antigen or aberrant immune checkpoint expression, modified microenvironment, or functionally altered innate or adaptive immune cells. [48]). In consequence, the bone marrow microenvironment fosters a protective niche for malignant cells, and leukemia-driven alterations in immune cells contribute to escape from immunosurveillance, resulting in leukemia progression. These escape mechanisms often necessitate treatment adaptation or combination therapies to counter the evolving leukemic cells. [18,49–52].
Composition and function of uncultured (leukemia-specific) immune cells in AML, ALL, CLL
Compared to immunoreactive cells without LAA (UNSTIM) stimulation, frequencies of many uncultured and antigen leukemia-specific (degranulating or IFNγ-producing) immunoreactive cells with (STIM) vs. without LAA stimulation (UNSTIM) were higher in AML, ALL, CLL patients´ and healthy donors´ samples (Fig.1.(1)). These data confirm that LAA stimulation increases the provision of leukemia-specific immunoreactive (uncultured) cells in AML. [11,12,37], but as we show here also in ALL and CLL. We also confirm that the addition of SEB in WB of healthy volunteers significantly increased the degranulation activity in healthy T cells, T3+, Tnon-naïve, and Tem/eff, as shown before [11,37] (Fig.1.D).
DC-Based Immunotherapy
Ex vivo, DC/DCleu subtypes can be generated from AML or ALL patients´ monocytes using combinations of response modifiers (GM-CSF, PGE1, IL-4, CD40L, TNFα) and loaded with leukemic antigens. [21–35] And DCleu can migrate to tissues in the whole body, present the patients’ entire leukemic antigen repertoire in a costimulatory manner to immune cells, and induce leukemia-specific cytotoxic lymphocytes, thereby providing an antileukemic strategy in the whole body [6,11,35,53]. An elegant way to improve DC-based immunotherapies (using DC/DCleu produced under GMP, followed by adoptive transfer to patients [6,10]) is to directly produce DC and DCleu in vivo (from monocytes or blasts) in the patients, resulting in activation of (leukemia-specific/ antileukemic) immune cells. Ex vivo, this strategy can be simulated using (heparinized) patients´ WB containing the patients´ complete cellular or soluble (potentially inhibitory or immune activating composition- as shown for AML samples treated with Kit-M or other Kits before (e.g., Schwepcke et al., 2021 [5], Unterfrauner et al., 2023 [37]).
Here, we confirm this Kit-M-mediated antileukemic approach for AML samples: Kit-M-pretreated leukemic WB produces leukemia-derived DC, which later increases leukemia-specific/ antileukemic cells after MLC (Fig.2.B; Fig.3.B). We can also note that Kit-M pretreatment of AML samples with versus without aberrant lymphoid marker expression yields comparable frequencies of DC subtypes, as well as immune cell subtypes, after MLC (Fig.2.E, F; Fig. 3E, F). Recently, we could show that generation of DC and DCleu from AML-WB using Kit-M is independent of patients´ ELN risk, mutation, HLA status, age, or sex [38]. Treating leukemia-diseased rats and therapy-refractory AML patients with these clinically approved drugs (GM-CSF and PGE1) in Kit-M, we could demonstrate that the treatment is safe, improves anti-leukemic responses in vivo, and improves hematopoietic recovery without increasing blast counts [39,57]. Previous findings show that DC and DCleu subtypes can be produced from AML-WB with Kits containing GM-CSF, combined, e.g. with PGE1 (Kit-M), with Picibanil (Kit-I), or with IFNα (Kit-E) -with Kit-M being the most effective Kit to induce antileukemic reactions against AML [5,12]. Here we can add in addition that `lymphoid` Kits (Kit-1 containing CD40L and IL-4 or Kit-2 containing GM-CSF, TNFα, and IL-2) are partially able to produce DC/DCleu subtypes-followed by induction of leukemia-specific or antileukemic immune cells from AML-WB.
Studying the influence of response modifiers in various combinations on the generation of DC/DCleu and DC/DCleu mediated leukemia-specific immune reactions in ALL-WB, we confirm data demonstrated before[29,32], that DC/DCleu can be generated with combined “lymphoid” Kit-1 and Kit-2 without inducing blast/monocyte proliferation. Especially, with Kit-1-pretreatment, we found the highest frequencies of DCleu/BLA (Fig.2.A). In addition, Kit-1 and Kit-2 are effective in generating DC/DCleu from ALL blasts, followed by antileukemic immunoactivation after T cell-enriched MLC [29,30,32]. Moreover, we can show that Kit-M is also able to produce DC and DCleu from ALL-WB, followed by antileukemic immunoactivation after T cell-enriched MLC, although with less efficiency compared to Kit-1 or Kit-2 (Fig.3.A). These data show that ALL is a stem cell disease with comparable cellular differentiation capabilities to AML [54,55]. In CLL patients´ WB samples, DC/DCleu could be generated under Kit-1-, Kit-2, and Kit-M-pretreatment with less success and in fewer cases (Fig.2.C). This could indicate that CLL (characterized by mature monoclonal B cells) may be less influenced by Kits´ treatment, whereas those affecting stem cell diseases may respond more effectively. Treating healthy donors´ WB samples with various Kits, we confirm previous findings that DC/DCleu can be generated with Kit-M-pretreated (vs. not pretreated) healthy patients’ WB samples without inducing monocyte proliferation (Fig.2.D) [11,35]. For future applications, Kit-M could be used to induce DC and DCleu in vivo, inducing leukemia-specific immune activation, which could lead to a stabilization of remission in AML and ALL patients. Kit-M is composed of approved drugs that can be used for clinical applications- a phase 1 trial for AML patients is in preparation.[39]. The clinical use of Kit-1 or Kit-2 drugs to treat ALL patients is not possible, since the components are not approved for clinical use.
Enhanced provision and activation of leukemia-specific immune cells after Kit-1/2 and Kit-M pretreatment of AML/ALL/CLL patients’ WB
We confirm preliminary data that Kit-M-treated (vs. untreated) AML-WB leads to the provision of activated and Tcm cells (Fig.3.B) [10,12]. In addition, we showed a generally higher activation status of immune cells after MLC in ALL patients´ Kit-1-pretreated (vs. untreated) WB, leading to increased frequencies of proliferating Tnon-naïve and Tcm (Fig.3.A). Memory T cells are critical for immune surveillance and the enhancement of anti-leukemic immunity, playing a key role in detecting and eliminating residual leukemia cells post-treatment, thereby improving treatment outcomes in ALL [52,56]. In CLL patients´ samples, Kit-1-pretreatment showed (non-significantly) increased frequencies of activated or Tcm cells (Fig.3.C).
Enhanced Intracellular Cytokine Production and Degranulation of Immune Cells after MLC in Kit-1-treated ALL patients´ WB and in Kit M-treated AML patients´ WB.
In AML samples, we confirmed previous studies indicating that Kit-M treatment of WB enhances anti-leukemic response after MLC. This includes an increase in IFNγ production by various T cell subtypes (e.g., IFNγ+Tnon-naïve, IFNγ+ Tem/eff, and IFNγ+Tcm). Additionally, we observed increased degranulation activity (CD107a expression) in these T cell subtypes (e.g., deg+Tnon-naïve, deg+Tem/eff ) and of NK cells (deg+NK); Furthermore, Kit-M treatment resulted in decreased frequencies of degranulating Treg in MLCWB-DC(Kit-M) compared to MLCWB-DC(Control), consistent with previous findings. (Fig.4.B) [35,37]. Through stimulation of immunoreactive cells using DC/DCleu-Kit-1 in ALL samples, we observed an increase in IFNγ-producing and degranulating adaptive and innate immune cells (e.g., IFNγ-producing Tnon-naïve, Tcm, NK, and CIK cells) in MLCWB-DC(Kit-1) compared to MLCWB-DC(Control), and an increase in degranulating T- or B-cell (e.g., deg+T3+ and deg+B) subtypes (Fig.4.A). These findings support the notion that Kit-1 treatment enhances the functional activity of adaptive immunity. Notably, previous studies have indicated that ALL-derived DCs might serve as a vaccine platform capable of eliciting LAA-specific T-cell responses. [30]. Our findings further substantiate this concept by demonstrating that Kit-1-treated ALL samples exhibit increased IFNγ production upon DC/DCleu stimulation, highlighting the activation of functionally competent leukemia-specific immune cells.
Importantly, in both ALL and AML samples, the production of IFNγ and other immunological markers was independent of patients’ age, sex, or blast frequency. This underscores the broad applicability of DC/DCleu-based/inducing strategies in inducing leukemia-specific immune responses across diverse patient subgroups. In CLL samples, we similarly observed increased IFNγ production of T cell subtypes (e.g., IFNγ+Tnon-naïve, IFNγ+Tem/eff and IFNγ+Tcm), as well as enhanced degranulation activity (CD107a expression) of T cells (e.g., deg+T3+, deg+Tcm), and (non-significantly) reduced frequencies of degranulating Treg (Fig.4.C). Overall, our data demonstrate increased antileukemic immunological activity in innate and adaptive immune compartments across all tested leukemia subtypes, further supporting the potential of Kit-based treatments to induce functionally active leukemia-specific immune cells.
Improved Blastolytic Activity after MLC in Kit-M-treated AML patients´ WB and Kit-1-treated ALL patients´ WB
In AML samples, we confirmed previous studies indicating that a higher number of cases with improved blast lysis was achieved after MLC with Kit-M-pretreated vs. untreated WB, following 3 or 24 hours of co-incubation of blast targets with effector cells (vs. Control), and also compared to Kit-1 or Kit-2 pretreated (Fig.5.B(1)) [11,13,34,35]. Blast lysis was superior in some cases after 3 hours of incubation of targets with effector cells, and in other cases after 24 hours in Kit-M-pretreated WB (Fig.5.B(2)). These effects may be attributed to distinct, independent blastolytic mechanisms: the faster perforin/granzyme pathways, which lead to blast lysis predominantly after 3 hours of co-incubation, and the slower Fas/FasL pathways, resulting in blast lysis predominantly after 24 hours of co-incubation [35]. In ALL samples, we could demonstrate a significantly improved lytic activity against leukemic blasts in WB through Kit-1, followed by Kit-2 and Kit-M mediated pretreatment (Fig.5.A(1)). Kit-1 pretreatment of blasts in WB has previously been demonstrated to enhance the antileukemic activation of immunoreactive cells, as evidenced by the induction of apoptosis in leukemic cells. [29,30]. Blast lysis was superior in some cases after 3 hours of incubation of the target with effector cells and in other cases after 24 hours in Kit-1-pretreated WB (Fig.5.A(2)). In CLL samples, we also observed a higher number of cases with improved blast lysis achieved after MLC with Kit-1-(followed by Kit-M and Kit-2) pretreated WB compared to untreated WB, following 3 or 24 hours of incubation of blast targets with effector cells (vs. Control) (Fig.5.C(1)), furthermore, blast lysis was superior in some cases after 3 hours of incubation of targets with effector cells, and in other cases after 24 hours in Kit-1-pretreated WB (Fig.5.C(2)), the sample size was too small to draw definitive conclusions about the effectiveness of Kits treatments.
Conclusion
We treated AML, ALL, CLL patients and healthy donors´ WB ex vivo with Kit-1 (IL-4 and CD40L), Kit-2 (GM-CSF, IL-4 and TNFα) or Kit-M (GM-CSF and PGE-1). We showed that DCleu-generation was best in AML patients ´WB with Kit-M (followed by Kit-1 and Kit-2) and in ALL patients´ WB with Kit-1(followed by Kit-M and Kit-2). However, both Kits were effective in AML and ALL and can induce leukemia-specific antileukemic immune effector and memory responses. Kit-pretreatment of leukemic WB not only activates antileukemic T cells, but also B, NK, and CIK cells, thereby improving the overall antileukemic response. Nonetheless, DC-inducing Kits applied in vivo to patients with myeloid or lymphoid leukemia, aiming at the conversion of (residual) blasts to leukemia-derived DCs (without the need of GMP procedures), appears to be promising, to avoid relapse by induced antileukemic effector and memory cells, since DC/DCleu can migrate to blood, bone marrow or tissues and prevent (extramedullary) relapses. Novel immunotherapeutic protocols should be developed that consider the roles of adaptive and innate immune cells subtypes in the cross-talk involved in DC/DCleu-triggered antileukemic responses, followed by integration in new immunotherapeutic strategies for leukemia treatment.
Author Contributions: X.F. and M.U. performed a great portion of the experiments. X.F. analyzed all flow cytometric and statistical data. S.B., P.A., L.L., H.A.R., A.H., T.B., J.A., A.S. contributed data to the DC, MLC, CTX, and DEG/INTCYT experiments, which were evaluated by X.F. P.B., D.K., J.S., C.S., G.F.V. provided the patients’ samples and reports. H.S. was responsible for the study design. X.F. contributed to the drafting of the manuscript. X.F. and H.S. contributed to editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding: The project was supported by intramural funding from the working group of H.S. The funders did not influence the study design, data collection, or analysis; the publishing decision; or the manuscript preparation.
Institutional Review Board Statement: Samples were collected and patients’ informed consent gathered according to the Helsinki guidelines and with the vote of the Ethics Committee of LMU in Munich (vote number: 33905).
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: The data presented in this study are available in this article.
Acknowledgments: The authors thank the patients, nurses, and physicians for their work and support with the sample material and diagnostic reports, and the technicians of the leukemia laboratory of the Medical Department III for platelet counting. The results presented in this article are part of the doctoral thesis of Xiaojia Feng at the Ludwig Maximilian University of Munich.
Conflicts of Interest: H.S. is involved with Modiblast Pharma GmbH (Oberhaching, Germany), which holds the European Patent 15 801 987.7-1118 and US Patent 15-517627, “Use of immunomodulatory effective compositions for the immunotherapeutic treatment of patients suffering from myeloid leukemias”. Moreover, a new application (as a priority claim) has been filed at the DPMA (German Patent and Trade Mark Office) to claim.
References
- Shah B, Mattison RJ, Abboud R, et al. Acute Lymphoblastic Leukemia, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw 22 (2024): 563–576.
- Pollyea DA, Altman JK, Assi R, et al. Acute Myeloid Leukemia, Version 3.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw 21 (2023): 503–513.
- Mohamed Jiffry MZ, Kloss R, Ahmed-Khan M, et al. A review of treatment options employed in relapsed/refractory AML. Hematology 28 (2023): 2196482.
- Shadman M. Diagnosis and treatment of chronic lymphocytic leukemia: a review. JAMA 329 (2023): 918–932.
- Schwepcke C, Klauer LK, Deen D, et al. Generation of leukaemia-derived dendritic cells (DCleu) to improve anti-leukaemic activity in AML: selection of the most efficient response modifier combinations. J. Mol. Sci. 23 (2022).
- van Acker HH, Versteven M, Lichtenegger FS, et al. Dendritic cell-based immunotherapy of acute myeloid leukemia. J. Clin. Med. 8 (2019).
- Zagorulya M, Spranger S. Once upon a prime: DCs shape cancer immunity. Trends Cancer 9 (2023): 172–184.
- Vogt V, Schick J, Ansprenger C, et al. Profiles of activation, differentiation markers, or β-integrins on T cells contribute to predicting T cells' antileukemic responses after stimulation with leukemia-derived dendritic cells. J. Immunother. 37 (2014): 331–347.
- Yu J, Sun H, Cao W, et al. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp. Hematol. Oncol. 11 (2022): 3.
- Amberger DC, Schmetzer HM. Dendritic cells of leukemic origin: specialized antigen-presenting cells as potential treatment tools for patients with myeloid leukemia. Med. Hemother. 47 (2020): 432–443.
- Schutti O, Klauer L, Baudrexler T, et al. Effective and successful quantification of leukemia-specific immune cells in AML patients' blood or culture, focusing on intracellular cytokine and degranulation assays. J. Mol. Sci. 25 (2024).
- Rackl E, Li L, Klauer LK, et al. Dendritic cell-triggered immune activation goes along with the provision of (leukemia-specific) integrin beta 7-expressing immune cells and improved antileukemic processes. J. Mol. Sci. 24 (2022).
- Pepeldjiyska E, Li L, Gao J, et al. Leukemia derived dendritic cell (DCleu) mediated immune response goes along with reduced (leukemia-specific) regulatory T-cells. Immunobiology 227 (2022): 152237.
- Hodder A, Mishra AK, Enshaei A, et al. Blinatumomab for first-line treatment of children and young persons with B-ALL. J. Clin. Oncol. 42 (2024): 907–914.
- Röllig C. Gemtuzumab ozogamicin in AML: the next chapter. Blood 142 (2023): 1673–1674.
- Shadman M, Maloney DG. Immune therapy for chronic lymphocytic leukemia: allogeneic transplant, chimeric antigen receptor T-cell therapy, and beyond. Hematol. Oncol. Clin. North Am. 35 (2021): 847–862.
- Huang YH, Wan CL, Dai HP, et al. Targeted therapy and immunotherapy for T cell acute lymphoblastic leukemia/lymphoma. Ann. Hematol. 102 (2023): 2001–2013.
- Vago L, Gojo I. Immune escape and immunotherapy of acute myeloid leukemia. J. Clin. Invest. 130 (2020): 1552–1564.
- Constantino J, Gomes C, Falcão A, et al. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol. Res. 65 (2017): 798–810.
- Lichtenegger FS, Krupka C, Haubner S, et al. Recent developments in immunotherapy of acute myeloid leukemia. J. Hematol. Oncol. 10 (2017): 142.
- Tsuchiya T, Hagihara M, Shimakura Y, et al. The generation of immunocompetent dendritic cells from CD34+ acute myeloid or lymphoid leukemia cells. Int. J. Hematol. 75 (2002): 55–62.
- Mohty M, Isnardon D, Blaise D, et al. Identification of precursors of leukemic dendritic cells differentiated from patients with acute myeloid leukemia. Leukemia 16 (2002): 2267–2274.
- Harrison BD, Adams JA, Briggs M, et al. Stimulation of autologous proliferative and cytotoxic T-cell responses by "leukemic dendritic cells" derived from blast cells in acute myeloid leukemia. Blood 97 (2001): 2764–2771.
- Köhler T, Plettig R, Wetzstein W, et al. Cytokine-driven differentiation of blasts from patients with acute myelogenous and lymphoblastic leukemia into dendritic cells. Stem Cells 18 (2000): 139–147.
- Osman Y, Takahashi M, Zheng Z, et al. Activation of autologous or HLA-identical sibling cytotoxic T lymphocytes by blood derived dendritic cells pulsed with tumor cell extracts. Oncol. Rep. 6 (1999): 1057–1063.
- Cignetti A, Bryant E, Allione B, et al. CD34+ acute myeloid and lymphoid leukemic blasts can be induced to differentiate into dendritic cells. Blood 94 (1999): 2048–2055.
- Choudhury A, Liang JC, Thomas EK, et al. Dendritic cells derived in vitro from acute myelogenous leukemia cells stimulate autologous, antileukemic T-cell responses. Blood 93 (1999): 780–786.
- Kremser A, Dressig J, Grabrucker C, et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: an evaluation of different methods. J. Immunother. 33 (2010): 185–199.
- Łuczyński W, Kowalczuk O, Iłendo E, et al. CD40L and IL-4 stimulation of acute lymphoblastic leukemia cells results in upregulation of mRNA level of FLICE--an important component of apoptosis. Folia Histochem. Cytobiol. 45 (2007): 15–20.
- Luczynski W, Kowalczuk O, Stasiak-Barmuta A, et al. Acute lymphoblastic leukemia-derived dendritic cells express tumor associated antigens: PNPT1, PMPCB, RHAMM, BSG and ERCC1. Neoplasma 56 (2009): 428–434.
- Narita M, Takahashi M, Liu A, et al. Generation of dendritic cells from leukaemia cells of a patient with acute promyelocytic leukaemia by culture with GM-CSF, IL-4 and TNF-alpha. Acta Haematol. 106 (2001): 89–94.
- Lim JH, Park CJ, Kim MJ, et al. Generation of lymphocytes potentiated against leukemic lymphoblasts by stimulation using leukemic cell lysate-pulsed dendritic cells in patients with acute lymphoblastic leukemia and measurement of in vitro anti-leukemic cytotoxicity. Hematology 17 (2012): 15–22.
- Tong XM, Yao HP, Qian WB, et al. The biological characteristics of dendritic cells derived in vitro from myelogeneous leukemia cells and healthy donor cells. Int. J. Lab. Hematol. 30 (2008): 372–381.
- Plett C, Klauer LK, Amberger DC, et al. Immunomodulatory kits generating leukaemia derived dendritic cells do not induce blast proliferation ex vivo: IPO-38 as a novel marker to quantify proliferating blasts in acute myeloid leukaemia. Clin. Immunol. 242 (2022): 109083.
- Klauer LK, Schutti O, Ugur S, et al. Interferon gamma secretion of adaptive and innate immune cells as a parameter to describe leukaemia-derived dendritic-cell-mediated immune responses in acute myeloid leukaemia in vitro. Transfus. Med. Hemother. 49 (2022): 44–61.
- Hirn Lopez A, Deen D, Fischer Z, et al. Role of interferon (IFN)α in "cocktails" for the generation of (leukemia-derived) dendritic cells (DCleu) from blasts in blood from patients with acute myeloid leukemia (AML) and the induction of antileukemic reactions. J. Immunother. 42 (2019): 143–161.
- Unterfrauner M, Rejeski HA, Hartz A, et al. Granulocyte-macrophage-colony-stimulating-factor combined with prostaglandin E1 create dendritic cells of leukemic origin from AML patients' whole blood and whole bone marrow that mediate antileukemic processes after mixed lymphocyte culture. Int. J. Mol. Sci. 24 (2023).
- Klauer LK, Rejeski HA, Ugur S, et al. Leukemia-derived dendritic cells induce anti-leukemic effects ex vivo in AML independently of patients' clinical and biological features. Int. J. Mol. Sci. 26 (2025).
- Atzler M, Baudrexler T, Amberger DC, et al. In vivo induction of leukemia-specific adaptive and innate immune cells by treatment of AML-diseased rats and therapy-refractory AML patients with blast modulating response modifiers. Int. J. Mol. Sci. 25 (2024).
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127 (2016): 2391–2405.
- Bahia DM, Yamamoto M, Chauffaille MdL, et al. Aberrant phenotypes in acute myeloid leukemia: a high frequency and its clinical significance. Haematologica 86 (2001): 801–806.
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140 (2022): 1345–1377.
- Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. Leukemia 9 (1995): 1783–1786.
- Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Semin. Hematol. 46 (2009): 64–75.
- Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 94 (2019): 1266–1287.
- Alfen JS, Larghi P, Facciotti F, et al. Intestinal IFN-γ-producing type 1 regulatory T cells coexpress CCR5 and programmed cell death protein 1 and downregulate IL-10 in the inflamed guts of patients with inflammatory bowel disease. J. Allergy Clin. 142 (2018): 1537–1547.e8.
- Aktas E, Kucuksezer UC, Bilgic S, et al. Relationship between CD107a expression and cytotoxic activity. Immunol. 254 (2009): 149–154.
- Sauerer T, Velázquez GF, Schmid C, et al. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: immune escape mechanisms and current implications for therapy. Mol. Cancer 22 (2023): 180.
- Cerreto M, Foà R, Natoni A, et al. The role of the microenvironment and cell adhesion molecules in chronic lymphocytic leukemia. Cancers (Basel) 15 (2023).
- Bakhtiyari M, Liaghat M, Aziziyan F, et al. The role of bone marrow microenvironment (BMM) cells in acute myeloid leukemia (AML) progression: immune checkpoints, metabolic checkpoints, and signaling pathways. Cell Commun. Signal. 21 (2023): 252.
- Taghiloo S, Asgarian-Omran H. Current approaches of immune checkpoint therapy in chronic lymphocytic leukemia. Curr. Treat. Options Oncol. 24 (2023): 1408–1438.
- Pastorczak A, Domka K, Fidyt K, et al. Mechanisms of immune evasion in acute lymphoblastic leukemia. Cancers (Basel) 13 (2021).
- Grabrucker C, Liepert A, Dreyig J, et al. The quality and quantity of leukemia-derived dendritic cells from patients with acute myeloid leukemia and myelodysplastic syndrome are a predictive factor for the lytic potential of dendritic cells-primed leukemia-specific T cells. J. Immunother. 33 (2010): 523–537.
- Pospísilová D, Borovicková J, Poloucková A, et al. Generation of functional dendritic cells for potential use in the treatment of acute lymphoblastic leukemia. Cancer Immunol. Immunother. 51 (2002): 72–78.
- Maggio R, Peragine N, Calabrese E, et al. Generation of functional dendritic cells (DC) in adult acute lymphoblastic leukemia: rationale for a DC-based vaccination program for patients in complete hematological remission. Lymphoma 48 (2007): 302–310.
- Li Y, Yang X, Sun Y, et al. Impact of T-cell immunity on chemotherapy response in childhood acute lymphoblastic leukemia. Blood 140 (2022): 1507–1521.
- Velázquez GF, Anand P, Abdulmajid J, et al. Clinical stabilization of a highly refractory acute myeloid leukaemia under individualized treatment with immune response modifying drugs by in vivo generation of dendritic cells of leukaemic origin (DCleu) and modulation of effector cells and immune escape mechanisms. Biomarker Res. 13 (2025): 104.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license 4.0
Supplementary Material
Patients’ Characteristics
We examined WB samples from 12 patients with AML, 11 patients with ALL, 7 patients with CLL, and 7 healthy volunteers. The female-to-male ratio in AML patients 2:1, in ALL patients was 1:1.37, in CLL patients 1:1.16 and in healthy volunteers 2.5:1. On average, AML patients were 61 (range 29–98), ALL patients were 48 (range 23–77), CLL patients were 74 (range 59–86), and healthy volunteers were 40 (range 25–60) years old.
AML patients’ samples were characterized using the WHO classification [40] and assigned to primary (pAML), secondary AML (sAML), or therapy-related AML (tAML) and subdivided according to the stage of the disease (first diagnosis, relapse after SCT). We further sorted the AML samples into two groups: blasts expressing aberrantly lymphoid markers, such as CD19, CD20, and CD3 (AML-Ly), and blasts without lymphoid marker expression (AML-Myo) [41]. The cytogenetic risk stratification (in favorable, intermediate, and adverse risks according to the European Leukemia Network (ELN) guideline 2022 [42]. The subtypes of the 11 ALL patients were immunologically classified according to the European Group of Immunophenotyping of Leukemias classification (EGIL) [43]: pro B-ALL (BI: n = 3), cALL (BII: n = 7), pre T-ALL (n = 1). Risk stratification of adult ALL patients was based on the Study Group for Adult Acute Lymphoblastic Leukemia (GMALL) as “standard” (n = 5) or “high risk” (n = 6) [44]. CLL patients (n=7) with persisting disease were analyzed. Risk stratification was based on the Binet classification: Binet A (n=7) [45]. Blast counts and phenotypes, as evaluated by flow cytometry in WB, are given for all patients; molecular mutations and numerical and structural chromosomal aberrations were collected on the day of sampling. An overview is given in Table 2.
The cellular composition of blood samples from patients with AML, the blood samples showed 28.75 (range 2–94) %blasts, 15.69 (range 4.58–23.89) %T cells, 5.7 (range 1.55–13.13) %NK cells, and 2.01 (range 0.22–7.2) %CIK cells. In patients with ALL, contained 33.18 (range 2–61) %, 14.27 (range 4.63–40.5) %T cells, 4.78 (range 1.83–10.33) %NK cells, and 2.57 (range 0.3–6.03) %CIK cells. For patients with CLL, the blood samples contained 57 (range 17–80) %blasts, 9.75 (range 1–22.5) %T cells, 3.16 (range 0.47–7.65) %NK cells, and 3.6 (range 0.13–14.79) %CIK cells. In healthy volunteers, the blood samples contained 4.86 (range 1.19–8.07) %monocytes, 15.03 (range 7.17–20.39) %T cells, 6.72 (range 2.15–12.23) %NK cells, and 3.87 (range 0.68–8.03) %CIK cells. In cases of aberrant expression of lineage markers on blasts, these markers were not used to quantify lymphocytes.
Flow Cytometry
Panels with various monoclonal antibodies (moAbs) labelled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin cyanin 7 (PCy7) or allophycocyanin (APC) were used, provided by Beckman Coultera (Krefeld, Germany), Becton Dickinsonb (Heidelberg, Germany), Bio Legendc (Amsterdam, Netherlands), Miltenyi Biotecd (Bergisch Gladbach, Germany), and Santa Cruz Biotechnologye (Heidelberg, Germany). Cells were stained with FITC-conjugated moAbs CD3b, CD4b, CD25a, CD33a, CD34a, CD71a, CD107c, CD117c, CD154b, CD45ROa, IPO38e; PE-conjugated moAbs CD3d, CD4b, CD56a, CD80a, CD117a, CD127a, CD206a, IFNγc; PC7-conjugated moAbs CD5a, CD15b, CD19a, CD3a, CD4a, CD25b, CD33a, CD34a, CD56a, CD117a, CD197b and APC- conjugated moAbs CD20a, CD10a, CD19a, CD3a, CD33a, CD34a, CD56a, CD80c, CD117a, CD14a, CD206b, CD45ROc. Non-viable cells were detected using 7AADb. Isotype controls were conducted according to the manufacturer’s instructions. Before staining, erythrocytes in WB samples were lysed with lysing buffer (Becton Dickinson). Cells were then incubated with the corresponding moAbs for 15 min in the dark using a staining medium containing 95% PBS and 5% FCS (Biochrom, Berlin, Germany). Intracellular staining (IPO38, IFNγ) was conducted with the FIX&PERMTM Cell Fixation and Permeabilization Kit (Thermo Fisher Scientific, Darmstadt, Germany).
Preparation of Samples
AML, ALL, CLL, or healthy WB was directly used for the setup of dendritic cell cultures and to perform the Degranulation Assay and the Intracellular Cytokine Assay. Peripheral blood mononuclear cells (PBMC) were isolated from the WB using the Ficoll-Hypaque density gradient centrifugation (Biocoll separating solution, Biochrom, Berlin, Germany). Afterwards, PBMC were used for the isolation of T cells via MACS microbead technology, based on a CD3 magnetic cell selection (CD3 Microbeads, Miltenyi Biotech, Bergisch Gladbach, Germany), as described in the manufacturer’s instructions. PBMC and T cells were frozen with 70% RPMI-1640 (Biochrom) containing 100 U/mL penicillin and 0.1 mg/mL streptomycin (PAN-Biotech, Aidenbach, Germany) (RPMI/PS), 20% human serum (PAN-Biotech), and 10% dimethyl sulfoxide (Sigma Aldrich Chemie GmbH, Steinheim, Germany) and stored at −80 oC until use, as shown before [35].
Dendritic Cell Culture (DCC)
WB cultures were incubated for 7–8 days under physiological conditions (37 oC, 5% CO2, 21% O2, and 95% humidity). Flow cytometric analyses of DC-subtypes and proliferating blasts from Kit-1-pretreated WB (DCWB(Kit-1) ), Kit-2-pretreated WB (DCWB(Kit-2) ), Kit-M-pretreated WB (DCWB(Kit-M) ), and untreated WB (DCWB(Control) ) were performed before and after culture using a refined gating strategy, as shown before [34,35].
T Cell-Enriched Mixed Lymphocyte Culture (MLC)
1x106 T cells and a fraction of DCC containing 2.5x105 DC/DCleu were co-cultured in 24-multiwell-tissue-culture-plates, with the total volume of the cell cultures being adjusted to 1ml with RPMI-1640-medium (Biochrom) containing 100U/ml penicillin (Biochrom). 50U/ml IL-2 (Peprotech) were added to all cultures (MLCWB-DC(Kit-1, Kit-2, Kit-M, Control)) on day 0 and day 2-3. Under physiological conditions (37 oC, 5% CO2, 21% O2, and 95% humidity), the MLC was incubated for 7 days, and measurements of Kit-treated samples were carried out: Kit-1 (MLCWB-DC(Kit-1)), Kit-2 (MLCWB-DC(Kit-2)), Kit-M (MLCWB-DC(Kit-M)), and Control (MLCWB-DC(Control)).
Intracellular Cytokine Assay (INTCYT)
The cells were stained with a PE-conjugated INFγ antibody (BioLegend, San Diego, CA, USA). Uncultured AML/ALL/CLL samples were stimulated with LAA: 2 μg/mL “Wilms Tumor 1” (PepTivator®WT1, Miltenyi Biotec) and 2 μg/mL “Preferentially Expressed Antigen of Melanoma” (PepTivator®PRAME, Miltenyi Biotec). Healthy samples were stimulated/not stimulated with 10 μg/mL staphylococcal enterotoxin B (SEB, Sigma-Aldrich, St. Louis, MO, USA). Unstimulated WB served as a control. To avoid spontaneous cytokine secretion, 5 μg/mL Brefeldin A solution (Bio Legend) was added, and the cultures were incubated for 16 h at 37 oC, 21% O2, and 10% CO2. After harvest, cells were centrifuged, resuspended in PBS/FCS, stained with antibodies, and analyzed by flow cytometry, as shown before [11].
Degranulation Assay (DEG)
A FITC-conjugated antibody against CD107a (Bio Legend, San Diego, CA, USA) was used to detect cell degranulation as a marker of cell cytotoxicity [46,47]. In analogy to the INTCYT, only uncultured ALL/AML/CLL samples were stimulated with LAA. Healthy samples were stimulated/not stimulated with 10 μg/mL SEB. Unstimulated WB served as a control. To avoid loss or weakening of CD107a antibodies’ fluorescence, 2 μg/mL Monensin solution (BioLegend) was added to the cultures. After an incubation of 16 h at 37 oC, 21% O2, and 10% CO2, cells were harvested, stained, and analyzed by flow cytometry, as shown before [11,13].
Cytotoxicity Fluorolysis Assay (CTX)
A fraction of MLCWB-DC (Kit-1, Kit-2, Kit-M ) and MLCWB-DC(Control) containing 1x106 T cells (effector cells) were co-cultured with 1x106 thawed autologous leukaemic blasts (target cells) for 3 and 24h at 37°C, 21% O2, 5% CO2. Target cells were stained with respective antibodies before incubation. After harvest, 7AAD and a defined number of Fluorosphere beads (Beckman Coulter) were added. The lytic activity of effector cells was calculated and defined as the percentage of viable target cells in the culture with co-cultured effector and target cells (for 3h and 24h), as compared to the Control. As a control, effector and target cells were cultured separately and mixed shortly before measurements.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 