Management of Venous Thromboembolism After Hip and Knee Arthroplasty
Alexander Postajian, Edgmin Rostomian, Alexander Abdou, Vedi Hatamian, Samson Keshishian, Devendra K. Agrawal*
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California, 91766, USA
*Corresponding Author: Devendra K. Agrawal, Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California, 91766, USA.
Received: 19 June 2025; Accepted: 27 June 2025; Published: 07 July 2025
Article Information
Citation:
Alexander Postajian, Edgmin Rostomian, Alexander Abdou, Vedi Hatamian, Samson Keshishian, Devendra K. Agrawal. Management of Venous Thromboembolism After Hip and Knee Arthroplasty. Journal of Orthopedics and Sports Medicine. 7 (2025): 311-327.
View / Download Pdf Share at FacebookAbstract
Venous thromboembolism (VTE), a term encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), remains a leading cause of mortality following total hip arthroplasty (THA) and total knee arthroplasty (TKA). Optimizing thromboprophylaxis, or the prevention of VTE after surgery, is becoming increasingly critical as the demand and frequency of total joint arthroplasty rises globally. This review covers the current literature on the risk factors, detection, and prevention of VTE in patients undergoing THA and TKA. It compares the efficacy and safety profiles of the most common pharmacological interventions including aspirin, low molecular weight heparin, rivaroxaban, apixaban, and warfarin. Mechanical methods, such as pneumatic compression devices, as well as intraoperative considerations, such as anesthesia modality, operative time, and tourniquet time, are also discussed. Furthermore, this review explores recent surgical advancements including minimally invasive approaches and robotic-assisted THA and TKA. Despite advancements and extensive research, the optimal thromboprophylaxis regimen remains debated, which highlights the need for individualized, patient-centered approaches to thromboprophylaxis.
Keywords
Deep vein thrombosis; Minimally invasive surgery; Pharmacological thromboprophylaxis; Pulmonary embolism; Robotic assisted surgery; Total joint arthroplasty; Total knee arthroplasty; Total hip arthroplasty; Venous thromboembolism
Deep vein thrombosis articles; Minimally invasive surgery articles; Pharmacological thromboprophylaxis articles; Pulmonary embolism articles; Robotic assisted surgery articles; Total joint arthroplasty articles; Total knee arthroplasty articles; Total hip arthroplasty articles; Venous thromboembolism articles
Article Details
1. Introduction
Total joint arthroplasty (TJA) is a commonly performed procedure designed to restore function and relieve pain in damaged or diseased joints by replacing them with prosthetic components. This intervention is most performed to address severe arthritis, fracture and degenerative joint diseases that impair quality of life [1]. Among the various types of joint replacement surgery, total hip arthroplasty and total knee arthroplasty are the most performed with osteoarthritis (OA) being the primary indication [2,3]. Despite the incredible success rates of these procedures, they do not come without risks.
Deep vein thrombosis (DVT), often leading to pulmonary embolism, remains one of the most common and serious complications associated with total joint arthroplasty and other conditions [4-10]. In THA specifically, the rate of lower extremity DVT has been reported to be as high as 19.78% and as low as 1.02% of cases [11,12]. This variation highlights the broad range of patient-related risk factors associated with postoperative DVT. Given these risks, the anticipated growth in the number of joint arthroplasties performed further underscores the importance of effective DVT prevention. Some studies predict the number of revision total hip arthroplasty (rTHA) procedures to increase by 42% by 2040 and 101% by 2060, as well a rise in revision total knee arthroplasty (rTKA) procedures by 149% by 2040 and 520% by 2060 [13-18]. This rise is mainly due to an aging population globally with an increase in life expectancy accompanied by increased prevalence of OA.
Given the rising demand of THA and TKA, addressing the associated risk of venous thromboembolism (VTE) is crucial to optimizing patient outcomes. Research efforts are focused on pharmacological, mechanical, and surgical methods for thromboprophylaxis [19]. This review aims to provide an overview of the current thromboprophylaxis measures that can be taken after total joint arthroplasty, with a particular focus on knee and hip arthroplasty, as well as to highlight current advancements in this area.
2. Surgical Background
Joint arthroplasty is generally divided into two categories: total joint arthroplasty (TJA) and partial joint arthroplasty, or hemiarthroplasty (HA) [20]. Total joint replacement entails removing all components of the damaged joint and replacing them with prosthetic materials. For example, total hip arthroplasty (THA) involves the replacement of the acetabulum of the pelvis as well as the femoral head, including parts of the proximal femoral shaft. When the acetabular cup does not need replacement, the procedure is then considered HA [21,22]. The choice of TJA over HA depends on patient-specific factors, including underlying pathology and long-term functional goals. Arthroplasties are further categorized into primary and revision arthroplasty, where primary revision refers to the first arthroplasty a patient receives in their lifetime while revision arthroplasty involves operating and modifying an existing prosthesis either because of implant wear over time or post-primary arthroplasty complications.
The materials used in prosthetics are crucial as the goal of joint replacement is for the implant to last as long as possible. There is potential for the body to reject implants which is mitigated by careful selection of the materials used to make prosthetic joint components. Most implants are made of metals such as titanium alloys and cobalt chromium [3]. The prosthetic components for THAs specifically are the ball and socket, which replace the femoral head the acetabular cup respectively, as well as the stem, which is a rod attached to the femoral component that fits within the medullary cavity of the femur, providing stability and proper weight distribution throughout the joint.
Equally important is the method of fixation whereby the prosthetic joint components are fixed in place allowing them to work effectively as a joint. With modern arthroplasty, the most common form of fixation has become cemented fixation, whereby polymethylmethacrylate (PMMA) cement is used to hold the implant in place [3]. Conversely, uncemented fixation relies on bone growth to permanently attach the prosthetic components to bone. Such prosthetics have recently undergone major advancements and are typically designed with specific surface components or materials that promote this bone growth process, such as porous metals [22-24]. Lastly, hybrid fixation integrates both techniques for maximal benefits, specifically in TKA whereby the tibial component is cemented, and the femoral component is not [25,26]. This fixation technique has shown to reduce post-operative loosening of prosthetic components, which is a common cause for revision arthroplasty. Ultimately, the best method of fixation depends on the type of procedure being done and specific patient factors, such as age [24,27]. For example, the benefits of cementless prosthesis include reduced operating time, increased bone stock preservation and improved integration of the implant, especially in younger and more active patients. However, regarding THA in patients aged 65 and older, several studies demonstrate lower rates of the need for revision arthroplasty after 1-2 years in patients who underwent cemented THA compared to the uncemented group, indicating decreased postoperative prosthetic complications [27,28]. Although rare, bone cement is associated with postoperative DVT through a phenomenon known as bone cement implantation syndrome [29-31]. However, this is typically not the sole reason to avoid cemented implants, as this decision relies on other patient-related factors.
3. Risk Factors and DVT Detection
Virchow’s triad is a commonly used definition of the factors that lead to venous thromboembolism and clarifies why TJA increases the risk for developing venous thrombosis. This triad includes endothelial damage, hypercoagulability, and venous stasis [32-34]. Endothelial damage refers to trauma inflicted to the endothelial layer lining blood vessels, which can occur in veins during major orthopedic surgery such THA and TKA [32]. Furthermore, surgery and other trauma tends to put the body into a hypercoagulable state, increasing the risk for thrombus formation [32,35]. Lastly, venous stasis can occur due to damage inflicted to the valves within veins during surgery as well as tourniquet use [36,37]. Venous stasis also occurs postoperatively because of temporary immobility caused by TJA, which is why early mobility following surgery is ideal for DVT prevention [26]. Any condition that increases one of the three factors in Virchow’s triad can theoretically increase the risk for developing DVT. There are several patient-related factors that further increase the risk for developing VTE following TJA, as shown in Figure 1.
3.1 Age
The most obvious of these factors is advanced age (>60 years) [36]. This is explained by the fact that venous compliance and fibrinolysis decreases with age while venous wall thickness increases [38]. On average, elderly individuals are also less mobile, increasing venous stasis, and have a higher risk of cardiovascular diseases that increase the risk of VTE.
3.2 Obesity
While the association of obesity with VTE after TJA is not very well established, there is some evidence to suggest that obesity increases the risk of VTE [39]. A study by Sloan et al. [40] found an increased risk of VTE after THA and TKA in patients with a BMI of at least 25 kg/m [40]. The proposed reasoning behind this increase is that obesity is correlated with increased operative times, lower mobility after surgery and reduced effectiveness of intermittent pneumatic compression (IPC) devices.
3.3 Diabetes
A meta-analysis by Yang et al. [41] found a statistically significant increase in DVT incidence following TKA in diabetic patients [41]. The proposed explanation behind this is that diabetic patients have abnormal mechanisms of coagulation, hemostasis and fibrinolysis, which promotes thrombus formation [42].
3.4 Arthritis
While osteoarthritis and rheumatoid arthritis are among the primary indications for THA and TKA, they are both associated with an increased risk of DVT following surgery, highlighting the need for careful preoperative management [2,3,32,34,36,43,44]. Both forms of arthritis can lead to immobility, increasing venous stasis and thus the risk for VTE [36,43]. In addition, the inflammation associated with rheumatoid arthritis can cause endothelial damage as well as increased hypercoagulability by upregulating procoagulants while downregulating fibrinolysis and anticoagulants [34].
3.5 Cardiovascular disease
Cardiovascular diseases such as congestive heart failure, atrial fibrillation and coronary artery disease, are known to increase the risk of VTE because of increasing venous stasis, endothelial injury and hypercoagulation [12,45,46]. Additionally, patients with cardiovascular diseases often require anticoagulant and antiplatelet medications, which can complicate thromboprophylaxis involved with TKA or THA [47,48]. TJA patients must discontinue the use of antiplatelet therapy 7-10 days prior to surgery to minimize the risk of excessive intraoperative bleeding [47]. This can become highly problematic for patients on anticoagulants because prolonged periods without their medication increases the risk for thrombotic events, myocardial infarction or cerebrovascular accidents [49]. Therefore, preventing lower extremity DVT and concomitant PE in patients suffering from cardiovascular diseases is a significant challenge.
3.6 Detection of DVT
There are several preoperative precautions that can be taken to mitigate the risk of developing DVT after total joint arthroplasty. One of the most common markers used to predict probability of DVT is a preoperative D-dimer test, as current research shows that increased D-dimer levels are associated with hypercoagulability and thus a higher risk of thrombosis [36,37,50]. Guo et al. [43] found that a preoperative D-dimer level greater than 0.585 µg/mL was significantly associated with an increased risk of DVT in patients undergoing TKA [43]. When used alongside D-dimer levels, ultrasonography is also an effective and commonly used tool to screen for DVT prior to surgery or for diagnosing DVT postoperatively [12,36,43,51]. These screenings can be utilized more frequently in high-risk individuals to detect DVTs before they embolize and cause catastrophic effects.
Although not typically used for the detection and prediction of DVT in patients undergoing TJA, some recent studies suggest that hematocrit (HCT) levels can predict the risk of developing DVT. For instance, Cong et al. [52] found that a HCT level less than 40% was a risk factor for DVT in older patients with hip fractures, like the value of 33.5% found by Li et al. [53]. Though these studies are focused on elderly patients admitted for hip fractures, their results can possibly implicate the use of HCT for the prediction of DVT risk surrounding THA and TKA. Another marker that has received some consideration as an early DVT predictor is the concentration of thrombin antithrombin complex (TAT) [54,55]. Specifically, a recent study by Lin et al. [54] examined the levels of TAT in patients following TKA and TJA [54]. They found a TAT level of 49.47 ng/mL in patients that developed DVT postoperatively and a level of 20.70 ng/mL in non-DVT patients. Similar to HCT, TAT can therefore be a possible predictor of VTE in patients undergoing TJA.
3.7 Thromboprophylaxis
To minimize the risk of postoperative VTE, administration of some form of thromboprophylaxis therapy is common practice following TKA and THA. Each potential technique discussed in this review is depicted in Figure 2 below. Aiming for early mobilization of patients in conjunction with a pharmacological thromboprophylaxis agent has proven to be effective at DVT prevention [56]. However, there is not one gold-standard guideline for physicians to follow and consequently there exists some disagreement surrounding the ideal method for thromboprophylaxis. This is currently the greatest challenge in managing the risk of DVT following TJA. The two most common guidelines that orthopedic surgeons follow, as seen in Figure 3A, are centered around meta-analyses conducted by the American Academy of Orthopedic Surgeons (AAOS) and the American College of Chest Physicians (ACCP) [57,58].
One major difference between the two guidelines is the ideal mode of thromboprophylaxis. The AAOS guideline recommends the use of aspirin, low-molecular-weight heparin (LMWH), warfarin, or factor Xa inhibitors, such as rivaroxaban and apixaban [57]. The ACCP, on the other hand, primarily recommends LMWH as the ideal thromboprophylaxis agent following TJA because of its safety and efficacy, while recommending apixaban, rivaroxaban, aspirin, vitamin K antagonists (warfarin), or intermittent pneumatic compression devices as alternative treatments [58] (Figure 3B). The second significant difference between guidelines includes the timeline of thromboprophylaxis. While the AAOS does not specify a strict timeline for the initiation of treatment, it does recommend continuing prophylaxis for a minimum of 10-14 days postoperatively and extending this window to 35 days depending on patient risk factors [57]. The ACCP guideline also recommends the same duration and extension of thromboprophylaxis but recommends initiating LMWH treatment either 12 hours or more preoperatively or 12 hours more postoperatively [58]. Though these guidelines are helpful for physicians, there is still no consensus on the optimal thromboprophylaxis medication as they all have the potential to cause postoperative bleeding [59-61]. While aspirin is the most frequently used in the United States, its superiority over other options is not well established but has been a topic of extensive research in recent years.
Figure 3: (A) The flowchart depicts the most common approach and guideline to pharmacological thromboprophylaxis involved in total knee arthroplasty (TKA) and total hip arthroplasty (THA). (B) The strengths and weaknesses of using the anticoagulants that are discussed in the article, highlighting the importance of individualized care based on patient related risk factors. CrCl, creatinine clearance; DOACs, direct oral anticoagulants; INR, international normalized ratio; LMWH, low molecular weight heparin; MI, myocardial infarction; VTE, venous thromboembolism.
4. Pharmacological Methods
4.1 Aspirin
Aspirin is a popular choice for thromboprophylaxis as a monotherapy following THA and TKA because it is relatively cheap, well tolerated, does not require regular blood testing and exhibits minimal risks [62]. With direct oral anticoagulants becoming more common, there have been extensive efforts to reevaluate the efficacy and safety of aspirin compared to these drugs, such as rivaroxaban. Multiple meta-analyses of randomized clinical trials have shown equal effectiveness between Aspirin and other popular oral anticoagulants when comparing the rate of postoperative VTE in patients [62-68]. These studies determined safety by observing the rate of adverse events, such as bleeding, that occurred in patients while on treatment and found no statistically significant difference between aspirin and other treatments. In fact, the meta-analysis conducted by Matharu and colleagues [68] found lower rates of bruising in the Aspirin monotherapy group.
However, other studies showed conflicting results. The CRISTAL randomized trial specifically found symptomatic VTE in 3.45% and 1.82% of patients in the aspirin and enoxaparin treatment groups respectively [69]. These results and those of several other meta-analyses suggest that Aspirin is inferior in preventing VTE after TJA when compared to newer oral anticoagulants [69-72]. Though there are inconsistencies in the findings of Aspirin’s effectiveness, one common conclusion among most of these studies is that Aspirin is equally as safe, if not safer, than other common oral anticoagulants (rivaroxaban and apixaban) when considering the risk of adverse effects such as bleeding [70-74].
4.2 Low Molecular Weight Heparin (LMWHs)
LMWHs, the most common being enoxaparin, is the thromboprophylaxis agent recommended by the American College of Chest Physicians (ACCP) guidelines [58]. Compared to aspirin, there seems to be less debate surrounding the efficacy of LMWH, with several systematic reviews and meta-analyses establishing that it is as effective at preventing VTE as newer direct oral anticoagulants [75-80]. Colwell et al. [75] highlight that the ACCP analysis showed a DVT prevalence of 33% with LMWH after TKA, which is reduced by about 50% compared to those without thromboprophylaxis [58,75].
While LMWH, such as enoxaparin, are highly effective thromboprophylaxis agents, they are also associated with increased postoperative risks, namely bleeding [19,79,80,81,82]. While the ACCP recommends the use of LMWH, they also highlight the increased bleeding rate of 2.4% [58,75]. A study by Wang et al. [81] reviewing patients undergoing TKA and THA in the US found a statistically significant increase of surgical site bleeding in LMWH treated patients (6.2%) compared to those given Warfarin (2.1%) (OR=3.82, 95% confidence interval [CI], 2.64 to 5.52) [81]. Despite the effectiveness of LMWH, careful consideration should be taken before administration as a prophylactic, especially in high-risk patients such as those with hemorrhagic disorders or gastrointestinal bleeding [83,84]. Further reasoning against LMWH use includes the fact that it is administered via daily subcutaneous injection, which some patients dislike due to the pain and injection site bleeding. This may lead to poor compliance in some patients.
4.3 Rivaroxaban and Apixaban
Rivaroxaban and apixaban are direct oral anticoagulants belonging to the class of antithrombotic drugs known as factor Xa inhibitors [85]. These compounds are commonly used for thromboprophylaxis following TJA and work by blocking the activity of factor Xa in the coagulation cascade, thus preventing blood clot formation.
The use of rivaroxaban following TJA is supported by the ACCP as well as multiple other studies [58,85-89]. A randomized, double blind study by Eriksson et al. [86] comparing THA patients treated with 10mg of rivaroxaban and 40mg of enoxaparin daily found a DVT incidence of 1.1% and 3.7% respectively (RR:0.26; 95% confidence interval [CI], 1.5 to 3.7; P<0.001) [86]. Furthermore, a meta-analysis by Huang et al. [87] demonstrated that rivaroxaban is associated with a statistically significant decrease in symptomatic DVT, asymptomatic DVT, symptomatic VTE, and proximal and distal DVT when compared to enoxaparin [87].
While rivaroxaban has been found to be a highly effective choice for thromboprophylaxis, the major concern surrounding its use is the risk of bleeding associated with it. Several studies have established that the risk of major bleeding after administration of rivaroxaban following TJA is comparable to that of enoxaparin [90-93]. Infact, a recent meta-analysis by Piple et al. [90] found a statistically significant increase in bleeding associated with rivaroxaban use compared to enoxaparin in both TKA and THA patients (TKA: adjusted odds ratio [aOR] = 2.58, P < 0.001; THA: aOR 1.64, P < 0.001) [90,91]. Furthermore, some data suggests that rivaroxaban is associated with other wound complications such as periprosthetic joint infections [92,93]. These findings warrant careful consideration of patient-related factors when choosing rivaroxaban as a thromboprophylaxis treatment.
Rivaroxaban and apixaban are similar in terms of their efficacy and safety profile and are often studied together. A study by Russel and Huo comparing the efficacy and safety of rivaroxaban and apixaban to enoxaparin found rivaroxaban and apixaban to be superior in preventing DVT following TKA [94]. Furthermore, there were no significant differences in associated bleeding between the drugs. Despite such findings, there are some studies suggesting differences between rivaroxaban and apixaban. For example, a study by Gomez-Outez et al. [95] found rivaroxaban to be associated with a higher risk of bleeding than apixaban with the two showing a similar risk of VTE [95]. Another study found that rivaroxaban was superior to apixaban in preventing overall VTE up to 35 days after THA in 2431 patients [96]. Therefore, while apixaban and rivaroxaban show similar efficacy and safety when used for thromboprophylaxis, rivaroxaban may have a slight edge in terms of preventing VTE while also having a lower safety profile.
4.4 Warfarin
While warfarin is a recommended form of thromboprophylaxis by the ACCP and AAOS, many studies suggest that it is not superior to other anticoagulants and may be associated with higher risks of bleeding than other antiplatelet therapies, like aspirin [57,58,97-100]. A meta-analysis by He et al. [97] found that warfarin displays a lower effectiveness for VTE prophylaxis following THA and TKA when compared to other oral anticoagulants such as rivaroxaban [97]. Those using warfarin also exhibited a higher rate of bleeding events in the 35 days following surgery. Another study conducted by Tan et al. [79] found that aspirin thromboprophylaxis resulted in a lower rate of DVT, overall VTE and PE compared to warfarin in both high-risk and non-high-risk patients [79]. Several other studies have found that aspirin was as effective if not more effective than warfarin at preventing VTE with a lower risk of bleeding in TKA and THA patients [97,99,100]. In addition, warfarin requires frequent blood monitoring which is inconvenient and costly. For these reasons, warfarin is not a popular choice for thromboprophylaxis following THA and TKA (used in 10% of TJA cases) [60,101].
4.5 Individualized pharmacological management
The most effective thromboprophylaxis for patients undergoing a total joint arthroplasty will require individualized assessments of their bleeding risk. Figure 3B summarizes the benefits and drawbacks to each thromboprophylaxis agent based on patient-specific risk factors. The most important factors include advanced age, (>65 years) renal impairment with creatinine clearance less than 50 mL/min, a history of major bleeding, concurrent use of antiplatelet therapy, low hemoglobin levels (<100 g/L) or baseline of anemia, significant liver disease such as cirrhosis, and active cancer, particularly involving the gastrointestinal or urogenital tracts [102-105]. In patients who have one or more of these risk factors, anticoagulant use should be done so with great caution.
Aspirin, which is generally safe, should be avoided in patients who have high bleeding risk or patients who are on another anticoagulant at the time of the procedure. The ASH guidelines recommend suspending aspirin to reduce the risk of bleeding complications in patients who are at high risk for bleeding or on a second anticoagulant, unless there is a recent acute coronary event or coronary intervention [58]. Aspirin should also be used cautiously in patients with severe renal impairment defined by low creatinine clearance (<30 mL/min). It should be avoided in patients with mechanical heart valves who will need more potent anticoagulants for safety measures and patients with thrombotic antiphospholipid syndrome. It should also be used carefully in patients with high perioperative myocardial infarction risk as aspirin may be associated with an increased risk of nonfatal myocardial infarctions [58].
Additionally, Low-molecular-weight heparins (LMWHs), although effective, require dose reduction and careful monitoring in patients with severe renal impairment (Creatinine clearance <30 mL/min) [106,107]. It should be used cautiously in those with spinal or epidural anesthesia due to risk of spinal hematoma [108]. The first dose should be delayed until after the epidural catheter has been removed, and the catheter should be removed 8 hours after the last dose of LMWH has been administered. Furthermore, LMWH should be avoided in patients with hypersensitivity to LMWH or heparin [106,107].
Warfarin, a teratogenic substance contraindicated in pregnancy, should be cautiously used in patients with poor compliance and increased risk of bleeding [58,107]. This medication requires frequent monitoring and dose adjustments due to its narrow therapeutic window and many patients have trouble adhering to regular monitoring of international normalized ratio (INR). Patients who have a history of atrial fibrillation who are already on Warfarin should stop the agent 3 to 5 days before surgery and typically will not require a bridging therapy [109].
Bleeding risk is a major factor when considering thromboprophylaxis, particularly with the use of direct oral anticoagulants (DOACs). While DOACs are effective in preventing venous thromboembolism (VTE), they should be used cautiously in patients with the bleeding risk factors above. In patients with severe renal impairment (creatinine clearance <30 mL/min), severe hepatic impairment (Child-Pugh B or C), those on strong CYP3A4 and P-glycoprotein inhibitors, those with mechanical heart valves, those with baseline anemia or active cancer, thrombotic antiphospholipid syndrome, and in specific perioperative contexts with high-bleeding procedures, other anticoagulants should be given before considering DOACs [106,110]. Ultimately, anticoagulation therapy in the context of arthroplasty should be tailored to each patient’s unique clinical profile to maximize safety while effectively reducing the risk of venous thromboembolism [111].
4.6 Challenges and Future Direction in Pharmacological Thromboprophylaxis
Evidently, there is still much to learn about how these anticoagulants act as thromboprophylaxis agents following TKA and THA. Although newer, direct oral anticoagulants are convenient and effective at preventing VTE following TJA, they present a significant risk of bleeding. Given the current data, Aspirin seems to offer the lowest risk of bleeding, but whether it is as effective for thromboprophylaxis as drugs like rivaroxaban and apixaban is still not entirely certain. The differences in results between meta-analyses may be due to the heterogeneity within the individual studies chosen. For example, the dose administered, patient-related risk factors and follow-ups are some factors that are not controlled for between studies in many of the meta-analyses presented above. Therefore, making definitive generalizations about the most effective and safest method of thromboprophylaxis is challenging since the ideal prophylactic method depends on patient-related factors. A recent advancement in this field is the use of machine learning models to predict the risk of a patient developing VTE following TJA [112,113]. Although this field requires more development, these models may help physicians choose the best mode of thromboprophylaxis following THA and TKA, yielding better patient outcomes.
4.7 Mechanical Compression
Mechanical thromboprophylaxis refers to the use of intermittent pneumatic compression devices (IPCD) following TJA to prevent the development of VTE. Some observed benefits of mechanical compression are reduced lower extremity edema following TKA or THA as well as minimized risk for postoperative blood loss compared to treatments like enoxaparin [114-116]. Mechanical compression is recommended as an alternative treatment by the ACCP guidelines but is often used in conjunction with pharmacological thromboprophylaxis because its effectiveness in VTE prevention is not very well established [58]. A recent systematic review and meta-analysis found that IPCDs significantly reduced the risk of DVT and PE after surgery when compared to no thromboprophylaxis at all [117]. However, this study did not focus on patients who underwent TKA or THA specifically. Conversely, a study by Dietz et al. [118] found no significant difference in the rate of VTE in TJA patients receiving aspirin compared to those receiving a combination of aspirin and IPCD treatment [118]. They also point out the major challenge of compliance in patients using IPCD, stating that patient compliance within the first two weeks after hospital discharge was 51%. This is explained by the fact that the recommended daily usage of IPCDs is 18 hours, and it is not particularly comfortable for patients [58]. Therefore, IPCDs as a monotherapy for thromboprophylaxis following TJA should be carefully considered due to the lack of patient compliance and evidence supporting their efficacy. Further investigation is necessary to understand the effect of mechanical compression on the risk of VTE prevention following TKA and THA.
5. Intraoperative Thromboprophylaxis and Surgical Advancements
5.1 Operative time
Several studies have identified a correlation between increased operative times and increased risk for VTE following THA and TKA [12,39,119,120]. Yu et al. [12] specifically found that a surgery duration greater than 120 minutes was associated with a higher risk for postoperative DVT in patients undergoing THA [12]. This is explained by the fact that increased operative time increases the duration of venous stasis as well as the hypercoagulable state resulting from surgery, thereby increasing the risk for DVT according to Virchow’s triad [32,33]. Thus, efforts to reduce operative time without sacrificing quality can be beneficial in the prevention of VTE associated with TKA and THA.
5.2 Anesthetic modalities
One of the intraoperative factors that may have a significant effect on the risk of VTE incidence after TJA is the type of anesthesia used during the procedure. The common modalities of anesthesia used during TJA include general anesthesia (GA), spinal anesthesia, epidural anesthesia and combined spinal epidural anesthesia (CSEA). Although GA is used most frequently in TJA (57.9%), some evidence suggests that GA, when compared to the other modalities, is associated with increased postoperative complications and mortality [121,122]. Diulus et al. [123] found that the odds of experiencing DVT was higher in THA and TKA patients who were given GA compared to those given spinal anesthesia (OR = 3.9; 95% CI: 1.2 to 17.3; P =0.04) [123]. Another study determined that neuraxial anesthesia, which encompasses spinal, epidural, and CSEA modes, was associated with a decreased 30 day DVT risk in THA patients compared to a GA group (OR= 0.63; 95% CI=0.4-0.9; P=0.02) [124]. Although many studies suggest that neuraxial anesthesia is equally as safe or safer than GA, it is not entirely certain which form of neuraxial anesthesia is best [121,124]. A study by Nakamura et al. [125] comparing combined epidural/general anesthesia and spinal anesthesia alone in TKA and THA patients found a 48% higher incidence of VTE in the spinal anesthesia group [125]. These results suggest that epidural anesthesia may have an advantage over spinal anesthesia. Although more research is needed to determine the relationship between anesthetic modality and postoperative VTE risk, opting for neuraxial anesthesia whenever possible in high-risk patients may be beneficial for thromboprophylaxis.
5.3 Tourniquet use
The use of a tourniquet during surgery has been studied as an intraoperative risk factor for DVT following TJA [126-128]. Although tourniquets are commonly used during TKA to minimize intraoperative blood loss, this is not the case for THA since the femoral head and acetabulum are located very deep in the pelvis, making tourniquet application difficult and possibly dangerous to the surrounding structures [36,129-132]. A meta-analysis by Ahmed et al. [126] found a postoperative DVT incidence of 3.4% in TKA patients who used a tourniquet vs 1.5% in the non-tourniquet group [126]. Similarly, Huang et al. [127] observed an increased early post-operative hypercoagulable state and incidence of below-knee DVT in the tourniquet group [127]. Although the bloodless field and reduced blood loss offered by tourniquet use can be ideal, it is important to consider the association with postoperative DVT when planning a TKA [36,133]. When a tourniquet is used, a possibly effective precaution that can be taken is minimizing the length of tourniquet time [134]. A study by Zan et al. [134] determined that releasing the tourniquet immediately after the prosthetic components are implanted was associated with a significantly lower risk of proximal DVT when compared to releasing after the application of dressings (4.6% vs 12%). Although these results are promising, there are minimal studies that demonstrate similar results. Therefore, more extensive research in this is needed to establish the association between tourniquet time and DVT incidence.
5.4 Minimally invasive approaches and robotic-assisted surgery
Though conventional TJA has become an incredibly successful procedure, there have been major advancements in minimally invasive surgical (MIS) approaches to TKA and THA within the past two decades [135]. These procedures aim to replace the targeted joint while minimizing the size of the incision made and the amount of muscle dissection during surgery [135-142]. Currently, the most widely used MIS approaches in THA are the minimally invasive anterolateral approach, direct anterior approach, and piriformis-sparing approach which all have the common objective of sparing musculotendinous structures such as the tensor fascia lata, piriformis muscle, and the gluteus medius and minimus muscles [135-137]. The predominant MIS techniques for TKA are the quadriceps sparing approach and the mini-medial parapatellar approach [138-140]. The proposed benefits of these MIS techniques include sparing of the extensor mechanism by preserving quadriceps muscle function, as well as decreased loss of blood supply to the joint. However, most studies demonstrate that MIS techniques do not directly reduce the risk of DVT following THA or TKA [143-145]. Theoretically, minimizing muscle damage through MIS may translate to earlier mobilization following surgery, thereby reducing the risk for DVT development. However, this connection has not been established and requires more extensive research.
Another recent advancement in the field of arthroplasty is computer-assisted navigation and robotic-assisted (RA) joint replacement whereby surgeons can use computer systems to more effectively and precisely plan and perform TJA and revision arthroplasty. Studies have consistently shown that RA joint replacement improves precision and accuracy of the prosthetic placement [146-150]. Clinically speaking, however, whether there is a reduction in postoperative VTE compared to traditional arthroplasty is yet to be determined. The few existing studies which investigate this correlation have determined that the risk of developing DVT after robotic assisted TKA and THA is lower when compared to traditional THA and TKA [151,152]. While this is promising, far more evidence is necessary to determine the relationship between postoperative DVT and RA TJA. Additionally, RA arthroplasty carries some risks of its own such as pin loosening [153,154].
Both RA and MIS are not highly favored and have not replaced traditional joint arthroplasty yet due to several factors, one of which is that they reduce the surgical window making it difficult to cement prosthetics accurately [138,139]. They also increase operative time, which can increase the risk for DVT as mentioned in an earlier section [12,39,40,119,120,155]. Overall, these techniques require specialized machinery, tools, and training [133]. Given the limited evidence of substantial benefits, the investment in these advancements may not yet be justified.
6. Conclusion
The prevention of DVT and concomitant PE in patients undergoing THA or TKA is complex and multifactorial. Pharmacologically speaking, each agent discussed comes with trade-offs in terms of efficacy and risks. Therefore, pharmacological thromboprophylaxis selection should balance efficacy with bleeding risks.
Aspirin is currently a widely used option, due to its low cost, ease of use, favorable safety profile, especially when it comes to its relatively lower bleeding risk [62-74]. It performs comparably to other agents, in standard-risk patients, though its efficacy in high-risk individuals is debatable. According to the CRISTAL trial and various meta-analyses, Aspirin has been slightly less effective at lowering the symptomatic VTE rates in comparison to other mainstay treatment options such as LMWH, with research showing 3.45% incidence of VTE using aspirin compared to 1.82% using enoxaparin [70].
Low-molecular-weight heparins are effective and reliable, especially in the hospital settings. However, they are associated with higher rates of surgical site bleeding and should be used with caution, particularly in high bleeding risk individuals, those with comorbid hemorrhagic disorders or history of gastrointestinal bleeding [19,79-84]. Warfarin, while still included in some guidelines, is less favored today due to its narrower therapeutic range, need for monitoring, and comparatively higher risk of bleeding and VTE.
Direct oral anticoagulants like rivaroxaban and apixaban offer convenient dosing and relatively the strongest VTE protection in patients compared to other mainstay pharmacological interventions including LMWH, with the DVT incidence of 1.1% and 3.7% respectively [86]. Furthermore, rivaroxaban has been associated with a statistically significant decrease in symptomatic DVT, asymptomatic DVT, symptomatic VTE, and proximal and distal DVT when compared to enoxaparin [87]. However, the research proves to be conflicting when considering how high the risk of bleeding is with DOAC use, especially with TJA. Some studies show DOACs having an equivocal risk for bleeding when compared with LMWH [90-93]. In contrast, a recent meta-analysis performed on patients undergoing TKA and THA showed a statistically significant increase in bleeding associated with rivaroxaban in comparison to enoxaparin, with an odds ratio of 2.58 [90,91]. Among the two DOACS studied, rivaroxaban and apixaban, apixaban may offer a slightly better bleeding profile with similar efficacy. In addition to the potential higher bleeding risk, rivaroxaban may be associated with increased wound complications including periprosthetic joint infections [92,93]. While DOACS are highly effective, it is important to use proper clinical judgement when selecting the best candidates, considering individual comorbid conditions and higher bleeding risk factors.
In summary, aspirin remains the safest option regarding bleeding but may be less protective in high-risk scenarios. Rivaroxaban and apixaban are more potent but must be used cautiously due to bleeding complications. LMWH sits somewhere between these two options in accordance to bleeding risks, and warfarin is now used infrequently. Ultimately, the choice of agent should be tailored to the individual patient’s thrombotic and bleeding risks. As predictive tools evolve, personalized thromboprophylaxis strategies will likely play a larger role in improving patient outcomes.
Mechanical thromboprophylaxis and intraoperative factors further aid in VTE prevention. The use of intermittent pneumatic compression devices (IPCD) has shown the ability to reduce lower extremity edema and postoperative blood loss compared to anticoagulant use like enoxaparin and promote improved outcomes when used in conjunction with medications [114-116]. Their effectiveness as a sole treatment remains limited due to issues with patient compliance, particularly after discharge, as the daily usage up to 18 hours is recommended for it to be effective and this is not comfortable according to patients [58].
Anesthesia also contributes to VTE outcomes, with neuraxial techniques being associated with a lower risk of postoperative DVT than with general anesthesia. While tourniquet use in TKA provides a bloodless surgical field and reduced intraoperative blood loss, it has been linked to a higher incidence of postoperative DVT when compared to non-tourniquet procedures, highlighting the importance to reduce tourniquet times.
Surgical approaches and practices also play a significant role in VTE prophylaxis. Because increased intraoperative times are associated with hypercoagulable states and prolonged venous stasis, the focus on newer modalities include efficiency and as minimally invasive techniques as possible. Recent advancements such as minimally invasive and robotic-assisted approaches aim to reduce tissue trauma and improve prosthetic precision; yet their benefits in reducing VTE incidence remain inconclusive, as increased operative times and technical challenges may offset their potential advantages. Collectively, these findings underscore the need for further research into integrated thromboprophylaxis strategies that consider both pharmacological and mechanical interventions, as well as intraoperative techniques, to optimize outcomes for patients undergoing TJA.
7. Key Points
- • Total hip and knee arthroplasties are increasingly common procedures with high success rates but carry a significant risk of venous thromboembolism (VTE).
- • Deep vein thrombosis (DVT) and pulmonary embolism (PE) are major postoperative complications that require proactive prevention strategies.
- • Patient-specific risk factors such as age, obesity, diabetes, cardiovascular disease, and arthritis can elevate the likelihood of developing VTE after surgery.
- • Preoperative screening tools like D-dimer tests and ultrasound imaging are useful for identifying patients at high risk for DVT.
- • Aspirin is widely used in the U.S. for thromboprophylaxis due to its safety and low cost, but its effectiveness compared to other anticoagulants remains debated.
- • Low molecular weight heparin (LMWH) is highly effective but carries a higher risk of bleeding and may reduce patient compliance due to injection-based delivery.
- • Direct oral anticoagulants like rivaroxaban and apixaban are effective alternatives but also raise concerns over bleeding risks and wound complications.
- • Mechanical methods, such as intermittent pneumatic compression devices (IPCD), can help reduce VTE risk but face limitations in patient compliance.
- • Intraoperative factors like longer surgery duration, use of general anesthesia, and tourniquet application may increase DVT risk.
- • Emerging techniques such as robotic-assisted surgery and machine learning risk prediction models offer promising avenues for improving individualized thromboprophylaxis.
Funding: The research education and activities of DKA are supported by the R25AI179582 grant from the National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Competing interests: All authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication: All authors have read the manuscript and consented for publication.
References
- Hsu H, Siwiec RM. Knee Arthroplasty (Archived). In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023).
- Magnusson K, Scurrah K, Ystrom E, et al. Genetic factors contribute more to hip than knee surgery due to osteoarthritis - a population-based twin registry study of joint arthroplasty. Osteoarthritis Cartilage 25 (2017): 878-884.
- Seidlitz C, Kip M. Introduction to the Indications and Procedures. In: Bleß HH, Kip M, eds. White Paper on Joint Replacement: Status of Hip and Knee Arthroplasty Care in Germany. Berlin (Germany): Springer; (2018): 1-14.
- Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty 23 (2008): 139-145.
- Lopes LA, Agrawal DK. Thromboembolism in the Complications of Long COVID-19. Cardiol Cardiovasc Med 7 (2023): 123-128.
- Aabedi A, Fraix MP, Agrawal DK. Surgical interventions in Severe Osteoarthritis: Pros and Cons. J Orthop Sports Med 7 (2025): 169-178.
- Enni JBA, Agrawal DK. Application of Artificial Intelligence and its Subsets in Various Stages of Knee Arthroplasty from Pre-op to Post-op: An Overview. J Orthop Sports Med 7 (2025): 96-102.
- Eskandar T, Ahmed Z, Pan J, et al. The Decline of Lumbar Artificial Disc Replacement. J Spine Res Surg 6 (2024): 86-92.
- Supra R, Supra R, Agrawal DK. Surgical Approaches in Total Hip Arthroplasty. J Orthop Sports Med 5 (2023): 232-240.
- Werner JH, Rosenberg JH, Keeley KL, et al. Immunobiology of periprosthetic inflammation and pain following ultra-high-molecular-weight-polyethylene wear debris in the lumbar spine. Expert Rev Clin Immunol 14 (2018): 695-706.
- Pedersen AB, Sorensen HT, Mehnert F, et al. Risk factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Joint Surg Am 92 (2010): 2156-2164.
- Yu X, Wu Y, Ning R. The deep vein thrombosis of lower limb after total hip arthroplasty: what should we care. BMC Musculoskelet Disord 22 (2021): 547.
- Matharu GS, Culliford DJ, Blom AW, et al. Projections for primary hip and knee replacement surgery up to the year 2060: an analysis based on data from The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Ann R Coll Surg Engl 104 (2022): 443-448.
- Scheuing WJ, Reginato AM, Deeb M, et al. The burden of osteoarthritis: Is it a rising problem? Best Pract Res Clin Rheumatol 37 (2023): 101836.
- Shichman I, Askew N, Habibi A, et al. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2040-2060. Arthroplast Today 21 (2023): 101152.
- Dubin JA, Bains SS, Hameed D, et al. Projected volume of primary total joint arthroplasty in the USA from 2019 to 2060 [published correction appears in Eur J Orthop Surg Traumatol 34 (2024): 2671.
- Kim HS, Park JW, Moon SY, et al. Current and Future Burden of Periprosthetic Joint Infection from National Claim Database. J Korean Med Sci 35 (2020): e410.
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage 30 (2022): 184-195.
- Sun G, Wu J, Wang Q, et al. Factor Xa Inhibitors and Direct Thrombin Inhibitors Versus Low-Molecular-Weight Heparin for Thromboprophylaxis After Total Hip or Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J Arthroplasty 34 (2019): 789-800.e6.
- Schmitz PP, van Susante JLC, Sierevelt IN, et al. The outcomes of conversion of hemiarthroplasty to total hip arthroplasty, a systematic review and meta-analysis. Arch Orthop Trauma Surg 144 (2024): 2993-3001.
- Li X, Luo J. Hemiarthroplasty compared to total hip arthroplasty for the treatment of femoral neck fractures: a systematic review and meta-analysis. J Orthop Surg Res 16 (2021): 172.
- Bota NC, Nistor DV, Caterev S, et al. Historical overview of hip arthroplasty: From humble beginnings to a high-tech future. Orthop Rev (Pavia) 13 (2021): 8773.
- Kamath AF, Siddiqi A, Malkani AL, et al. Cementless Fixation in Primary Total Knee Arthroplasty: Historical Perspective to Contemporary Application. J Am Acad Orthop Surg 29 (2021): e363-e379.
- Uivaraseanu B, Vesa CM, Tit DM, et al. Highlighting the advantages and benefits of cementless total knee arthroplasty (Review). Exp Ther Med 23 (2022): 58.
- González Della Valle A, Sharrock N, Barlow M, et al. The modern, hybrid total hip arthroplasty for primary osteoarthritis at the Hospital for Special Surgery. Bone Joint J 98-B (2016): 54-59.
- Wang Z, Chen X, Zhou Y, et al. Hybrid fixation versus full-cemented or full-cementless fixation in total knee arthroplasty: Systematic review and meta-analysis of comparative studies. J Orthop Sci 25 (2020): 1047-1054.
- Hameed D, McCormick BP, Sequeira SB, et al. Cemented Versus Cementless Femoral Fixation for Total Hip Arthroplasty Following Femoral Neck Fracture in Patients Aged 65 and Older. J Arthroplasty 39 (2024): 1747-1751.
- Boyle AB, Zhu M, Frampton C, et al. Comparing modern uncemented, hybrid and cemented implant combinations in older patients undergoing primary total hip arthroplasty, a New Zealand Joint Registry study. Arch Orthop Trauma Surg 143 (2023): 3597-3604.
- Dahl OE, Pripp AH, Jaradeh M, et al. The Bone Cement Hypercoagulation Syndrome: Pathophysiology, Mortality, and Prevention. Clinical and Applied Thrombosis/Hemostasis 29 (2023).
- Saleem S, Odelugo C, Viswanathan S. A Hard Clot: Predisposition to Cement Pulmonary Embolism and Pulmonary Infection After Kyphoplasty [abstract]. Am J Respir Crit Care Me 205 (2022): A1639.
- Bhadani JS, Kumar I, Ahmed W, et al. Hemodynamic Effects of Bone Cement Implantation in Hip Arthroplasty: Insights from a Prospective Study in Eastern India. J Orthop Case Rep 14 (2024): 212-221.
- Nachman D, Pollack A, Herzog E. Epidemiology, Pathophysiology and Predisposing Factors of Pulmonary Embolism and Deep Vein Thrombosis. In: Herzog, E. (eds) Pulmonary Embolism. Springer, Cham (2022).
- Bereda G. Risk Factors, Diagnosis, Pathophysiology and Management of Deep Vein Thrombosis. J Clin Med Images Case Reports 2 (2022): 1-5.
- Omair MA, Alkhelb SA, Ezzat SE, et al. Venous Thromboembolism in Rheumatoid Arthritis: The Added Effect of Disease Activity to Traditional Risk Factors. Open Access Rheumatol 14 (2022): 231-242.
- Senst B, Tadi P, Basit H, et al. Hypercoagulability. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023).
- Gao JQ, Xue ZQ, Huang JY, et al. Risk of deep vein thrombosis (DVT) in lower extremity after total knee arthroplasty (TKA) in patients over 60 years old. Journal of Orthopaedic Surgery and Research 18 (2023): 865.
- Tsukada S, Saito M, Ogawa H, et al. Thrombotic events in patients underwent simultaneous bilateral total knee arthroplasty with modern perioperative blood management strategy. Arch Orthop Trauma Surg 145 (2024): 2.
- Akrivou D, Perlepe G, Kirgou P, et al. Pathophysiological Aspects of Aging in Venous Thromboembolism: An Update. Medicina (Kaunas) 58 (2022): 1078.
- Ervando H, Ridwan LS, Dilogo IH. Factors related to deep vein thrombosis as a complication of post-total hip arthroplasty patients: a systematic review [published correction appears in Eur J Orthop Surg Traumatol 35 (2025): 172.
- Sloan M, Sheth N, Lee GC. Is Obesity Associated with Increased Risk of Deep Vein Thrombosis or Pulmonary Embolism After Hip and Knee Arthroplasty? A Large Database Study. Clin Orthop Relat Res 477 (2019): 523-532.
- Yang G, Meng F, Liu Y, et al. Diabetes mellitus and risk of deep vein thrombosis after total knee replacement: a meta-analysis of cohort studies. Int J Clin Exp Med 8 (2015): 9086-9092.
- Jiao X, Li Z, An S, et al. Does diabetes mellitus increase the incidence of early thrombosis in deep vein following unicompartmental knee arthroplasty: a retrospective cohort study. BMC Geriatr 22 (2022): 448.
- Guo YF, Gao N, Chen Y, et al. Incidence of and risk factors for preoperative deep vein thrombosis in elderly patients with end-stage osteoarthritis following total knee arthroplasty: a retrospective cohort study. BMC Musculoskelet Disord 25 (2024): 754.
- Zeng C, Bennell K, Yang Z, et al. Risk of venous thromboembolism in knee, hip and hand osteoarthritis: a general population-based cohort study. Ann Rheum Dis 79 (2020): 1616-1624.
- Dai WL, Lin ZM, Shi ZJ, et al. Venous Thromboembolic Events after Total Knee Arthroplasty: Which Patients Are at a High Risk?. J Knee Surg 33 (2020): 947-957.
- Xiong X, Cheng B. Preoperative risk factors for deep vein thrombosis in knee osteoarthritis patients undergoing total knee arthroplasty. J Orthop Sci 28 (2023): 180-187.
- Wu CT, Lien TH, Chen IL, et al. The Risk of Bleeding and Adverse Events with Clopidogrel in Elective Hip and Knee Arthroplasty Patients. J Clin Med 11 (2022): 1754.
- Maxfield DG, Bernasek TL, Engel CC, et al. Is it Safe to Continue Clopidogrel in Elective Hip and Knee Arthroplasty?. J Arthroplasty 37 (2022): 1726-1730.
- Dang X, Liu M, Yang Q, et al. Tranexamic acid may benefit patients with preexisting thromboembolic risk undergoing total joint arthroplasty: a systematic review and meta-analysis. EFORT Open Rev 9 (2024): 467-478.
- Jiang T, Yao Y, Xu X, et al. Prevalence and Risk Factors of Preoperative Deep Vein Thrombosis in Patients with End-Stage Knee Osteoarthritis. Ann Vasc Surg 64 (2020): 175-180.
- Schellong SM, Beyer J, Kakkar AK, et al. Ultrasound screening for asymptomatic deep vein thrombosis after major orthopaedic surgery: the VENUS study. J Thromb Haemost 5 (2007): 1431-1437.
- Cong Y, Wang B, Fei C, et al. Dynamic observation and risk factors analysis of deep vein thrombosis after hip fracture. PLoS One 19 (2024): e0304629.
- Li DY, Lu DX, Yan T, et al. The Association between the Hematocrit at Admission and Preoperative Deep Venous Thrombosis in Hip Fractures in Older People: A Retrospective Analysis. J Clin Med 12 (2023): 353.
- Lin Z, Sun H, Li D, et al. Thrombin antithrombin complex concentration as an early predictor of deep vein thrombosis after total hip arthroplasty and total knee arthroplasty. BMC Musculoskelet Disord 23 (2022): 574.
- Ginsberg JS, Brill-Edwards P, Panju A, et al. Pre-operative plasma levels of thrombin-antithrombin III complexes correlate with the development of venous thrombosis after major hip or knee surgery. Thromb Haemost 74 (1995): 602-605.
- Husted H, Otte KS, Kristensen BB, et al. Low risk of thromboembolic complications after fast-track hip and knee arthroplasty. Acta Orthop 81 (2010): 599-605.
- Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg 19 (2011): 777-778.
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141 (2012): e278S-e325S.
- Glassberg MB, Lachiewicz PF. Changing Patterns of Anticoagulation After Total Hip Arthroplasty in the United States: Frequency of Deep Vein Thrombosis, Pulmonary Embolism, and Complications with Rivaroxaban and Warfarin. J Arthroplasty 34 (2019): 1793-1801.
- Simon SJ, Patell R, Zwicker JI, et al. Venous Thromboembolism in Total Hip and Total Knee Arthroplasty. JAMA Network Open 6 (2023): e2345883.
- Schelde AB, Petersen J, Jensen TB, et al. Thromboembolic and bleeding complications following primary total knee arthroplasty : a Danish nationwide cohort study. Bone Joint J 103-B (2021): 1571-1577.
- Za P, Papalia GF, Franceschetti E, et al. Aspirin is a safe and effective thromboembolic prophylaxis after total knee arthroplasty: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 31 (2023): 4407-4421.
- Hong Z, Su Y, Zhang L, et al. Aspirin Is as Effective and Safe as Oral Anticoagulants for Venous Thromboembolism Prophylaxis After Joint Arthroplasty: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Bone Joint Surg Am 107 (2025): 760-770.
- Major Extremity Trauma Research Consortium (METRC), O'Toole RV, Stein DM, et al. Aspirin or Low-Molecular-Weight Heparin for Thromboprophylaxis after a Fracture. N Engl J Med 388 (2023): 203-213.
- Anderson DR, Dunbar M, Murnaghan J, et al. Aspirin or Rivaroxaban for VTE Prophylaxis after Hip or Knee Arthroplasty. N Engl J Med 378 (2018): 699-707.
- Matharu GS, Kunutsor SK, Judge A, et al. Clinical Effectiveness and Safety of Aspirin for Venous Thromboembolism Prophylaxis After Total Hip and Knee Replacement: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern Med 180 (2020): 376-384.
- Lavu MS, Porto JR, Hecht CJ 2nd, et al. Low-Dose Aspirin Is the Safest Prophylaxis for Prevention of Venous Thromboembolism After Total Knee Arthroplasty Across All Patient Risk Profiles. J Bone Joint Surg Am 106 (2024): 1256-1267.
- Watts PJ, Kopstein M, Harkness W, et al. A retrospective analysis of bleeding risk with rivaroxaban, enoxaparin, and aspirin following total joint arthroplasty or revision. Pharmacotherapy 41 (2021): 608-615.
- CRISTAL Study Group, Sidhu VS, Kelly TL, et al. Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial. JAMA 328 (2022): 719-727.
- Liu HZ, Liang J, Hu AX. The efficacy and safety of aspirin in preventing venous thrombosis in major orthopedic surgery: An updated meta-analysis of randomized controlled trials. Medicine (Baltimore) 102 (2023): e35602.
- Salman LA, Altahtamouni SB, Khatkar H, Al-Ani A, Hameed S, Alvand A. The efficacy of aspirin versus low-molecular-weight heparin for venous thromboembolism prophylaxis after knee and hip arthroplasty: A systematic review and meta-analysis of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc 33 (2025): 1605-1616.
- Zheng X, Nong L, Song Y, et al. Comparison of efficacy and safety between aspirin and oral anticoagulants for venous thromboembolism prophylaxis after major orthopaedic surgery: a meta-analysis of randomized clinical trials. Front Pharmacol 14 (2024): 1326224.
- Cheok T, Beveridge A, Berman M, et al. Efficacy and safety of commonly used thromboprophylaxis agents following hip and knee arthroplasty. Bone Joint J 106-B (2024): 924-934.
- Shohat N, Ludwick L, Goh GS, et al. Aspirin Thromboprophylaxis Is Associated With Less Major Bleeding Events Following Total Joint Arthroplasty. J Arthroplasty 37 (2022): 379-384.e2.
- Colwell CW Jr, Hardwick ME. Rationale for low-molecular-weight heparin prophylaxis after total knee arthroplasty. Clin Orthop Relat Res 452 (2006): 181-185.
- Xia ZN, Zhou Q, Zhu W, et al. Low molecular weight heparin for the prevention of deep venous thrombosis after total knee arthroplasty: A systematic review and meta-analysis. Int J Surg 54 (2018): 265-275.
- Lu X, Lin J. Low molecular weight heparin versus other anti-thrombotic agents for prevention of venous thromboembolic events after total hip or total knee replacement surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord 19 (2018): 322.
- Meng J, Liu W, Xiao Y, et al. The role of aspirin versus low-molecular-weight heparin for venous thromboembolism prophylaxis after total knee arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg 109 (2023): 3648-3655.
- Tan TL, Foltz C, Huang R, et al. Potent Anticoagulation Does Not Reduce Venous Thromboembolism in High-Risk Patients. J Bone Joint Surg Am 101 (2019): 589-599.
- Shang J, Wang L, Gong J, et al. Low molecular weight heparin dosing regimens after total joint arthroplasty: a prospective, single-center, randomized, double-blind study.” Journal of Orthopaedic Surgery and Research 19 (2024): 799.
- Wang Z, Anderson FA Jr, Ward M, et al. Surgical site infections and other postoperative complications following prophylactic anticoagulation in total joint arthroplasty. PLoS One 9 (2014): e91755.
- Suen K, Westh RN, Churilov L, et al. Low-Molecular-Weight Heparin and the Relative Risk of Surgical Site Bleeding Complications: Results of a Systematic Review and Meta-Analysis of Randomized Controlled Trials of Venous Thromboprophylaxis in Patients After Total Joint Arthroplasty. J Arthroplasty 32 (2017): 2911-2919.e6.
- Patel P, Varacallo MA. Low-Molecular-Weight Heparin (LMWH). In: StatPearls. Treasure Island (FL): StatPearls Publishing (2025).
- Jupalli A, Iqbal AM. Enoxaparin. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023).
- Eriksson BI, Kakkar AK, Turpie AG, et al. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement [published correction appears in J Bone Joint Surg Br 91 (2009): 1120.
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358 (2008): 2765-2775.
- Huang HF, Li SS, Yang XT, et al. Rivaroxaban versus enoxaparin for the prevention of venous thromboembolism after total knee arthroplasty: A meta-analysis. Medicine (Baltimore) 97 (2018): e13465.
- Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost 105 (2011): 444-453.
- Etscheidt J, Shahien A, Gainey M, et al. Review of Therapeutic Options for the Prevention of VTE in Total Joint Arthroplasty. Geriatrics (Basel) 5 (2020): 18.
- Piple AS, Wang JC, Kang HP, et al. Safety and Efficacy of Rivaroxaban in Primary Total Hip and Knee Arthroplasty. J Arthroplasty 38 (2023): 1613-1620.e4.
- Ricket AL, Stewart DW, Wood RC, et al. Comparison of Postoperative Bleeding in Total Hip and Knee Arthroplasty Patients Receiving Rivaroxaban or Enoxaparin. Ann Pharmacother 50 (2016): 270-275.
- Brimmo O, Glenn M, Klika AK, et al. Rivaroxaban Use for Thrombosis Prophylaxis Is Associated With Early Periprosthetic Joint Infection. J Arthroplasty 31 (2016): 1295-1298.
- Jameson SS, Rymaszewska M, Hui AC, et al. Wound complications following rivaroxaban administration: a multicenter comparison with low-molecular-weight heparins for thromboprophylaxis in lower limb arthroplasty. J Bone Joint Surg Am 94 (2012): 1554-1558.
- Russell RD, Huo MH. Apixaban and rivaroxaban decrease deep venous thrombosis but not other complications after total hip and total knee arthroplasty. J Arthroplasty 28 (2013): 1477-1481.
- Gómez-Outes A, Terleira-Fernández AI, Suárez-Gea ML, et al. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. BMJ 344 (2012): e3675.
- Highcock AJ, As-Sultany M, Finley R, et al. A Prospective Cohort Comparative Study of Rivaroxaban, Dabigatran, and Apixaban Oral Thromboprophylaxis in 2431 Hip and Knee Arthroplasty Patients: Primary Efficacy Outcomes and Safety Profile. J Arthroplasty 35 (2020): 3093-3098.
- He T, Han F, Wang J, et al. Efficacy and safety of anticoagulants for postoperative thrombophylaxis in total hip and knee arthroplasty: A PRISMA-compliant Bayesian network meta-analysis. PLoS One 16 (2021): e0250096.
- Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines fomanagement of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 3 (2019): 3898-3944.
- Huang RC, Parvizi J, Hozack WJ, et al. Aspirin Is as Effective as and Safer Than Warfarin for Patients at Higher Risk of Venous Thromboembolism Undergoing Total Joint Arthroplasty. J Arthroplasty 31 (2016): 83-86.
- Singh G, Prentice HA, Winston BA, et al. Comparison of 90-Day Adverse Events Associated With Aspirin and Potent Anticoagulation Use for Venous Thromboembolism Prophylaxis: A Cohort Study of 72,288 Total Knee and 35,142 Total Hip Arthroplasty Patients. J Arthroplasty 38 (2023): 1602-1612.e1.
- Nam D, Sadhu A, Hirsh J, et al. The use of warfarin for DVT prophylaxis following hip and knee arthroplasty: how often are patients within their target INR range? J Arthroplasty 30 (2015): 315-319.
- Khan F, Tritschler T, Kimpton M, et al. Long-Term Risk for Major Bleeding During Extended Oral Anticoagulant Therapy for First Unprovoked Venous Thromboembolism: A Systematic Review and Meta-analysis. Ann Intern Med 174 (2021): 1420-1429.
- Gomez Lumbreras A, Tan MS, Moorman-Bishir K, et al. Risk of Bleeding Among Individuals on Direct-Acting Oral Anticoagulants: An Academic Medical Center Cohort Study. J Cardiovasc Pharmacol 80 (2022): 813-819.
- Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients with Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 73 (2021): 366-413.
- Olie RH, Winckers K, Rocca B, et al. Oral Anticoagulants Beyond Warfarin. Annu Rev Pharmacol Toxicol 64 (2024): 551-575.
- Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative Management of Antithrombotic Therapy: An American College of Chest Physicians Clinical Practice Guideline [published correction appears in Chest 164 (2023): 267.
- Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest 141 (2012): 1129. Dosage error in article text] [published correction appears in Chest. 2012 Dec;142(6):1698. Dosage error in article text. Chest 141 (2012): 7S-47S.
- Hirsh J, Anand SS, Halperin JL, et al. American Heart Association. AHA Scientific Statement: Guide to anticoagulant therapy: heparin: a statement for healthcare professionals from the American Heart Association. Arterioscler Thromb Vasc Biol 21 (2001): E9.
- Lieberman JR, Bell JA. Venous Thromboembolic Prophylaxis After Total Hip and Knee Arthroplasty. J Bone Joint Surg Am 103 (2021): 1556-1564.
- Bavalia R, Middeldorp S, Weisser G, et al. Treatment of Venous Thromboembolism in Special Populations with Direct Oral Anticoagulants. Thromb Haemost 120 (2020): 899-911.
- Muscatelli SR, Charters MA, Hallstrom BR. Time for an Update? A Look at Current Guidelines for Venous Thromboembolism Prophylaxis After Hip and Knee Arthroplasty and Hip Fracture. Arthroplast Today 10 (2021): 105-107.
- Shohat N, Ludwick L, Sherman MB. et al. Using machine learning to predict venous thromboembolism and major bleeding events following total joint arthroplasty. Sci Rep 13 (2023): 2197.
- Lex JR, Koucheki R, Abbas A, et al. Predicting 30-Day Venous Thromboembolism Following Total Joint Arthroplasty: Adjusting for Trends in Annual Length of Stay. Arthroplast Today 30 (2024): 101491.
- Carnevale Pellino V, Gatti A, Vandoni M, et al. Pneumatic Compression Combined with Standard Treatment after Total Hip Arthroplasty and Its Effects on Edema of the Operated Limb and on Physical Outcomes: A Pilot Clinical Randomized Controlled Study. J Clin Med 12 (2023): 4164.
- Maradei-Pereira JAR, Sauma ML, Demange MK. Thromboprophylaxis with unilateral pneumatic device led to less edema and blood loss compared to enoxaparin after knee arthroplasty: randomized trial. BMC Musculoskelet Disord 23 (2022): 984.
- Greenall R, Davis RE. Intermittent pneumatic compression for venous thromboembolism prevention: a systematic review on factors affecting adherence. BMJ Open 10 (2020): e037036.
- Kim NY, Ryu S, Kim YH. Effects of intermittent pneumatic compression devices interventions to prevent deep vein thrombosis in surgical patients: A systematic review and meta-analysis of randomized controlled trials. PLoS One 19 (2024): e0307602.
- Dietz MJ, Moushmoush O, Frye BM, et al. Randomized Trial of Postoperative Venous Thromboembolism Prophylactic Compliance: Aspirin and Mobile Compression Pumps. J Am Acad Orthop Surg 30 (2022): e1319-e1326.
- Zhang H, Mao P, Wang C, et al. Incidence and risk factors of deep vein thrombosis (DVT) after total hip or knee arthroplasty: a retrospective study with routinely applied venography. Blood Coagul Fibrinolysis 28 (2017): 126-133.
- Bohl DD, Ondeck NT, Darrith B, et al. Impact of Operative Time on Adverse Events Following Primary Total Joint Arthroplasty. J Arthroplasty 33 (2018): 2256-2262.e4.
- Johnson RL, Kopp SL, Burkle CM, et al. Neuraxial vs general anaesthesia for total hip and total knee arthroplasty: a systematic review of comparative-effectiveness research. Br J Anaesth 116 (2016): 163-176.
- Warren J, Sundaram K, Anis H, et al. Spinal Anesthesia Is Associated With Decreased Complications After Total Knee and Hip Arthroplasty. J Am Acad Orthop Surg 28 (2020): e213-e221.
- Diulus SC, Mucharraz C, Schmitt DR, et al. Morbidity and Mortality Following Total Hip and Knee Arthroplasty With Spinal Versus General Anesthesia: A Retrospective Analysis. J Arthroplasty 39 (2024): 2675-2679.
- Baldawi M, Awad ME, McKelvey G, et al. Neuraxial Anesthesia Significantly Reduces 30-Day Venous Thromboembolism Rate and Length of Hospital Stay in Primary Total Hip Arthroplasty: A Stratified Propensity Score-Matched Cohort Analysis. J Arthroplasty 38 (2023): 108-116.
- Nakamura M, Kamei M, Bito S, et al. Spinal anesthesia increases the risk of venous thromboembolism in total arthroplasty: Secondary analysis of a J-PSVT cohort study on anesthesia. Medicine (Baltimore) 96 (2017): e6748.
- Ahmed I, Chawla A, Underwood M, et al. Time to reconsider the routine use of tourniquets in total knee arthroplasty surgery. Bone Joint J 103-B (2021): 830-839.
- Huang CR, Pan S, Li Z, et al. Tourniquet use in primary total knee arthroplasty is associated with a hypercoagulable status: a prospective thromboelastography trial. Int Orthop 45 (2021): 3091-3100.
- Mori N, Kimura S, Onodera T, et al. Use of a pneumatic tourniquet in total knee arthroplasty increases the risk of distal deep vein thrombosis: A prospective, randomized study. Knee 23 (2016): 887-889.
- Quintero JI, Cárdenas LL, Navas M, et al. Primary Joint Arthroplasty Surgery: Is the Risk of Major Bleeding Higher in Elderly Patients? A Retrospective Cohort Study. J Arthroplasty 31 (2016): 2264-2268.
- Goker B, Caglar O, Kinikli GI, et al. Postoperative bleeding adversely affects total knee arthroplasty outcomes in hemophilia. Knee 39 (2022): 261-268.
- Lee JH, Han SB. Patient Blood Management in Hip Replacement Arthroplasty. Hip Pelvis 27 (2015): 201-208.
- Tsukada S, Saito M, Ogawa H, et al. Thrombotic events in patients underwent simultaneous bilateral total knee arthroplasty with modern perioperative blood management strategy. Arch Orthop Trauma Surg 145 (2024): 2.
- Oragui E, Parsons A, White T, et al. Tourniquet use in upper limb surgery. Hand (N Y) 6 (2011): 165-173.
- Zan P, Mol MO, Yao JJ, et al. Release of the tourniquet immediately after the implantation of the components reduces the incidence of deep vein thrombosis after primary total knee arthroplasty. Bone Joint Res 6 (2017): 535-541.
- Clesham K, Sheridan GA, Greidanus NV, et al. Minimally Invasive Intermuscular Approaches Versus Conventional Approaches in Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. J Arthroplasty 37 (2022): 1658-1666.
- Lalevée M, Curado J, Matsoukis J, et al. Comparative MRI assessment of three minimally invasive approaches in total hip arthroplasty. Orthop Traumatol Surg Res 108 (2022): 103354.
- Liu H, Yin L, Li J, et al. Minimally invasive anterolateral approach versus direct anterior approach total hip arthroplasty in the supine position: a prospective study based on early postoperative outcomes. J Orthop Surg Res 17 (2022): 230.
- Migliorini F, Eschweiler J, Baroncini A, et al. Better outcomes after minimally invasive surgeries compared to the standard invasive medial parapatellar approach for total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 29 (2021): 3608-3620.
- Yang X, Cheng QH, Yang YZ, et al. Minimally invasive medial femoral approach to total knee arthroplasty improves short-term outcomes compared to the standard medial parapatellar approach: a systematic review and meta-analysis. J Orthop Surg Res 18 (2023): 657.
- Zhang L, Li X, Rüwald JM, et al. Comparison of minimally invasive approaches and standard median parapatellar approach for total knee arthroplasty: A systematic review and network meta-analysis of randomized controlled trials. Technol Health Care 29 (2021): 557-574.
- Ge J, Hernigou P, Guo W, et al. Minimally invasive small incision surgical technique for unicompartmental knee arthroplasty. Int Orthop 47 (2023): 2717-2725.
- Yen SH, Lin PC, Wu CT, et al. Comparison of Effects of a Thrombin-Based Hemostatic Agent and Topical Tranexamic Acid on Blood Loss in Patients with Preexisting Thromboembolic Risk Undergoing a Minimally Invasive Total Knee Arthroplasty. A Prospective Randomized Controlled Trial. Biomed Res Int 2021 (2021): 2549521.
- Warren JA, Sundaram K, Anis HK, et al. Have Venous Thromboembolism Rates Decreased in Total Hip and Knee Arthroplasty?. J Arthroplasty 35 (2020): 259-264.
- Januel JM, Chen G, Ruffieux C, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA 307 (2012): 294-303.
- Migliorini F, Biagini M, Rath B, et al. Total hip arthroplasty: minimally invasive surgery or not? Meta-analysis of clinical trials. Int Orthop 43 (2019): 1573-1582.
- Deckey DG, Rosenow CS, Verhey JT, et al. Robotic-assisted total knee arthroplasty improves accuracy and precision compared to conventional techniques. Bone Joint J 103-B (2021): 74-80.
- Maman D, Laver L, Becker R, et al. Trends and epidemiology in robotic-assisted total knee arthroplasty: Reduced complications and shorter hospital stays. Knee Surg Sports Traumatol Arthrosc 32 (2024): 3281-3288.
- Alrajeb R, Zarti M, Shuia Z, et al. Robotic-assisted versus conventional total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Eur J Orthop Surg Traumatol 34 (2024): 1333-1343.
- Wu XD, Zhou Y, Shao H, et al. Robotic-assisted revision total joint arthroplasty: a state-of-the-art scoping review. EFORT Open Rev 8 (2023): 18-25.
- Rajan PV, Khlopas A, Klika A, et al. The Cost-Effectiveness of Robotic-Assisted Versus Manual Total Knee Arthroplasty: A Markov Model-Based Evaluation. J Am Acad Orthop Surg 30 (2022): 168-176.
- Maman D, Laver L, Becker R, et al. Robotic-assisted total knee arthroplasty reduces postoperative complications and length of stay without increased cost compared to navigation-guided techniques: A national analysis. Knee Surg Sports Traumatol Arthrosc 33 (2025): 336-342.
- Shah AK, Lavu MS, Burkhart RJ, et al. Robotic-assistance is associated with better joint outcomes compared to conventional techniques in surgically routine total hip arthroplasty: a propensity-matched large database study of 3948 patients. Arch Orthop Trauma Surg 145 (2025): 114.
- Thomas TL, Goh GS, Nguyen MK, et al. Pin-Related Complications in Computer Navigated and Robotic-Assisted Knee Arthroplasty: A Systematic Review. J Arthroplasty 37 (2022): 2291-2307.e2.
- Bukowski BR, Sandhu KP, Bernatz JT, et al. CT required to perform robotic-assisted total hip arthroplasty can identify previously undiagnosed osteoporosis and guide femoral fixation strategy. Bone Joint J 105-B (2023): 254-260.
- Alcelik IA, Blomfield MI, Diana G, et al. A Comparison of Short-Term Outcomes of Minimally Invasive Computer-Assisted vs Minimally Invasive Conventional Instrumentation for Primary Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J Arthroplasty 31 (2016): 410-418.
Related PubMed Articles
- Thromboembolic safety and clinical benefits of tranexamic acid beyond hemostasis in post-COVID-19 patients undergoing major arthroplasty: A STROBE-compliant retrospective study.
- The Safety of Patients Who Have Preoperative Venous Thromboembolism Undergoing Joint Arthroplasty.
- Management of Venous Thromboembolism After Hip and Knee Arthroplasty.
- Improving Patient Care and Clinical Services: Compliance With the National Institute for Health and Care Excellence (NICE) Guidelines for Venous Thromboembolism Prophylaxis After Hip and Knee Arthroplasty.
- Optimizing blood management in arthroplasty: a meta-analysis of carbazochrome sodium sulfonate and Tranexamic acid combination.
- Estrogen Replacement Therapy Decreases Associated Risk of Postoperative Venous Thromboemboli and Medical Complications After Total Joint Arthroplasty.
- Bleeding Complications, Transfusion, and Acute Care Costs After Major Arthroplasty in Patients With Hereditary Bleeding Disorders: A National Healthcare Database Analysis.
- Effects and Complications of Apixaban versus Aspirin for Venous Thromboembolism Prophylaxis after Total Hip or Knee Arthroplasty.
- [Modern Approach to the Use of Aspirin in Prevention of Venous Thromboembolism Following Total Hip Arthroplasty or Total Knee Arthroplasty. A Retrospective Trial].
- Comparing fondaparinux and low molecular weight heparin for thromboprophylaxis after hip and knee arthroplasty: a systematic review and meta-analysis.
- Letter to the editor: Effects of pre- and postsurgery physical activity interventions on physical activity and sedentary behavior levels following knee and hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials.
- Correction: Extracapsular hip fractures are more prone to perioperative thrombosis than intracapsular hip fractures in elderly patients undergoing arthroplasty for hip fractures: a retrospective cohort study.
- Image-Based Robotic Unicompartmental Knee Arthroplasty Results in Fewer Radiologic Outliers with No Impact on Revision Rates Compared to Imageless Systems: A Systematic Review.
- Review of Methods for Evaluating Changes in the Tension and Properties of the Gluteus Medius Muscle (GMED) and the Tensor Fascia Latae (TFL) as a Result of Hip Osteoarthritis (HOA) and After Total Hip Arthroplasty (THA)-Could MyotonPRO Assessment Be the N
- C-Reactive Protein to Albumin Ratio and Prognostic Nutrition Index as a Predictor of Periprosthetic Joint Infection and Early Postoperative Wound Complications in Patients Undergoing Primary Total Hip and Knee Arthroplasty.
- Associations Between Common Hip and Knee Osteoarthritis Treatments and All-Cause Mortality.
- Glycoprotein non-metastatic melanoma protein B is a potential biomarker for arthroplasty aseptic loosening.
- Lidocaine-Bupivacaine Spinal Proves Safe and Effective in Outpatient Total Joint Arthroplasty.
- Hip and Knee Arthroplasty in Patients with Obesity.
- Total Hip Arthroplasty with Cage Application for Complex Posterior Hip Dislocation and Acetabular Fracture in a Patient with Prior Patellectomy: A Case Report.

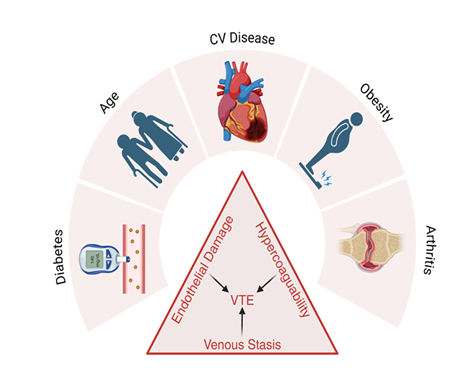
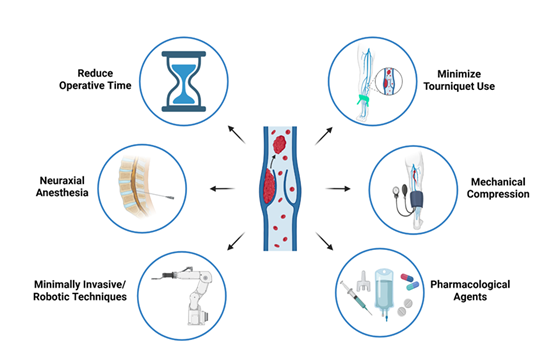
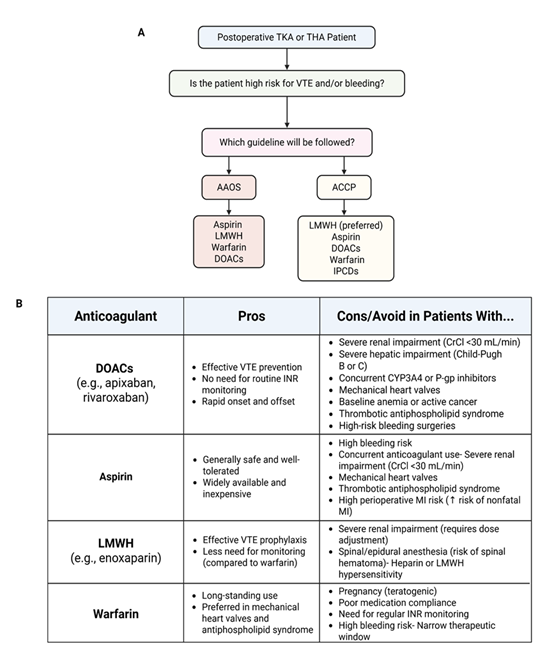

 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 