Plants, Pills, and the Brain: Exploring Phytochemicals and Neurological Medicines
Arnav Aggarwal1, Resmi Rajalekshmi2, Anshu Aggarwal3, Devendra K. Agrawal2*
1Loveless Academic Magnet Program, Montgomery, AL, USA
2Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
3Department of Biology and Environmental Sciences, College of Science, Auburn University at Montgomery, Montgomery, AL 36117, USA
*Corresponding Authors: Devendra K. Agrawal, Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California, 91766, USA.
Received: 07 July 2025; Accepted: 18 July 2025; Published: 04 August 2025
Article Information
Citation:
Arnav Aggarwal, Resmi Rajalekshmi, Anshu Aggarwal, Devendra K. Agrawal. Plants, Pills, and the Brain: Exploring Phytochemicals and Neurological Medicines. International Journal of Plant, Animal and Environmental Sciences 15 (2025): 90-114
View / Download Pdf Share at FacebookAbstract
Neurological disorders, such as Alzheimer’s disease, Parkinson’s disease, epilepsy, spinal cord injuries, and traumatic brain injuries, represent substantial global health challenges due to their chronic and often progressive nature. While allopathic medicine offers a range of pharmacological interventions aimed at managing symptoms and mitigating disease progression, it is accompanied by limitations, including adverse side effects, the development of drug resistance, and incomplete efficacy. In parallel, phytochemicals—bioactive compounds derived from plants—are receiving increased attention for their potential neuroprotective, antioxidant, and anti-inflammatory properties. This review will explore the therapeutic landscape of neurological diseases by providing a comprehensive overview of prevalent conditions and the current allopathic treatments available. Furthermore, this review will investigate specific phytochemicals, including flavonoids, alkaloids, and terpenoids, that exhibit promise in modulating various disease pathways. Emphasis will be placed on the interactions between plant-derived compounds and prescription medications, highlighting both potential synergistic effects and possible adverse interactions. A thorough understanding of these interactions is essential for the development of integrative treatment approaches that enhance therapeutic efficacy while minimizing harm. By bridging traditional herbal medicine with contemporary pharmacotherapy, this review aims to promote a more holistic perspective on the management of neurological diseases, while also encouraging further research into safe and effective combinatory therapies.
Keywords
<p>Alkaloids; Alzheimer’s disease; Epilepsy; Flavonoids; Neurodegenerative disease; Neurological disorders; Parkinson’s disease; Phytochemicals; Spinal cord injuries; Terpenoids; Traumatic brain injury</p>
Article Details
1. Introduction
Neurological diseases, as defined by the World Health Organization, are central and peripheral nervous disorders. The central nervous system includes the brain and spinal cord, while the peripheral nervous system is a network of nerves that extends throughout the head, neck, and body. These diseases can be grouped into several categories, such as neurodegenerative disorders (e.g., Alzheimer's and Parkinson's), neuromuscular conditions, brain disorders (like epilepsy), and spinal cord conditions. They often lead to progressive damage that affects memory, movement, and overall daily activity.
While allopathic medicine, conventional Western medical treatment, primarily uses pharmaceutical drugs and procedures to manage these conditions, it may not have long-term solutions or help address the underlying causes. In contrast, phytochemicals, naturally occurring compounds in plants known for their potential health benefits, have become of interest. These substances are found in fruits, vegetables, herbs, and other plant sources, which may influence brain function and protect nerve cells. It is essential to study how phytochemicals interact with modern allopathic treatment, as it may reveal complementary effects, reduce side effects, and help create more effective treatments for neurological diseases.
2. Overview of Neurological Disease
Neurological disorders encompass a wide range of conditions that affect the brain, spinal cord, and nerves. These diseases can be progressive, episodic, or chronic, and they often result in a combination of cognitive, motor, behavioral, and sensory impairments. Among the most prevalent and impactful neurological conditions are Alzheimer’s disease, Parkinson’s disease, epilepsy, and traumatic brain injury (Figure 1). Each of these disorders exhibits distinct etiologies and pathologies, yet many share overlapping features of neuronal damage, neuroinflammation, and neurodegeneration.
2.1 Alzheimer’s Disease
Alzheimer’s disease (AD) is the most prevalent form of dementia, accounting for an estimated 60–80% of cases globally. It is a progressive neurodegenerative condition characterized by memory loss, disorientation, and impaired reasoning. The hallmark pathological features include extracellular amyloid-beta (Aβ) plaque accumulation and intracellular neurofibrillary tangles formed from hyperphosphorylated tau protein. These abnormalities lead to synaptic dysfunction, neuronal death, and cerebral atrophy, particularly in the hippocampus and cortex [1].
Cognitive deficits are often preceded by mild cognitive impairment (MCI), which may progress over years. Neuroinflammation and oxidative stress are central to the pathogenesis, with microglial activation playing a dual role in both clearing amyloid and propagating damage. Genetic factors, such as mutations in APP, PSEN1/2, and the APOE ε4 allele, increase disease risk [2,3].
2.2 Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s, primarily affecting motor control. It is marked by the degeneration of dopaminergic neurons in the substantia nigra pars compacta, leading to dopamine deficiency in the striatum. Neuropathologically, PD is associated with Lewy bodies—cytoplasmic inclusions composed mainly of misfolded alpha-synuclein protein [4,5].
Clinical symptoms begin insidiously, with tremor at rest, bradykinesia, rigidity, and postural instability. Non-motor manifestations—including depression, anosmia, sleep disturbances, and cognitive impairment—often precede motor symptoms and can dominate the disease burden [6,7].
Although the etiology is multifactorial, genetic mutations in SNCA, LRRK2, and PINK1, as well as environmental exposures to toxins like pesticides, have been implicated. A growing body of evidence links gut microbiota and alpha-synuclein misfolding as early contributors to pathogenesis [8].
2.3 Epilepsy
Epilepsy is a neurological disorder marked by the tendency for recurrent, unprovoked seizures due to abnormal, excessive neuronal activity in the brain. It is a heterogeneous condition with multiple subtypes classified based on seizure type (e.g., focal, generalized), etiology, and electroclinical features [9].
Seizures can range from subtle lapses in consciousness (absence seizures) to dramatic convulsions involving the entire body (tonic-clonic seizures) [10]. Depending on the brain region involved, seizures may present with sensory disturbances, automatisms, motor abnormalities, or behavioral changes. The underlying causes of epilepsy include genetic mutations, brain injury, infections, developmental disorders, and metabolic conditions. In many cases, the etiology remains idiopathic [11].
2.4 Traumatic Brain Injury
Traumatic brain injury (TBI) results from an external mechanical force that causes brain dysfunction. TBIs can range from mild concussions to severe brain damage and are categorized based on the mechanism (blunt or penetrating), severity, and the affected brain region [12].
The pathophysiology of TBI involves both primary injury, which occurs at the moment of impact, and secondary injury, which evolves over time due to processes like cerebral edema, ischemia, excitotoxicity, and neuroinflammation [13,14]. TBI can cause diffuse axonal injury, contusions, hemorrhages, and disruption of the blood-brain barrier.
Clinical manifestations vary widely and may include loss of consciousness, confusion, amnesia, headache, dizziness, behavioral changes, and cognitive impairment. Severe TBIs are associated with long-term neurological deficits and an increased risk of developing neurodegenerative conditions like Alzheimer’s disease and Parkinson’s disease [15,16].
Additionally, repetitive mild TBIs—such as those sustained in contact sports—are linked to chronic traumatic encephalopathy (CTE), a degenerative brain disease marked by behavioral abnormalities, mood disturbances, and cognitive decline. Studies also show that TBI may precipitate late-onset psychiatric conditions and accelerate neurodegenerative processes [17].
3. Phytochemicals
Phytochemicals, also termed phytonutrients, encompass a structurally diverse group of secondary metabolites synthesized by plants via complex biosynthetic pathways. Unlike essential micronutrients, these compounds are not required for human survival but exhibit significant bioactive properties, including antioxidant, anti-inflammatory, antimicrobial, anticancer, and neuroprotective activities. Their diversity, spanning polyphenols, flavonoids, carotenoids, alkaloids, terpenoids, glucosinolates, and saponins, reflects extensive structural and functional heterogeneity [18].
Polyphenols form one of the most abundant and researched categories, including flavonoids, phenolic acids, stilbenes, and lignans. Flavonoids—such as flavonols (quercetin, kaempferol), flavanols (catechins), flavones, isoflavones, and anthocyanins—are particularly notable for their C6–C3–C6 structural motif that influences antioxidant behavior and interaction with cellular signaling pathways [19]. Anthocyanins, common in berries, red cabbage, and purple corn, contribute visual pigmentation and have demonstrated cardioprotective and anti-inflammatory properties [20].
Carotenoids, including β-carotene, lutein, and lycopene, are isoprenoid pigments that function in photoprotection and oxidative stress modulation. They are precursors to vitamin A and exhibit protective effects against chronic eye and cardiovascular diseases. Terpenes and terpenoids, structurally derived from five-carbon isoprene units, include compounds such as artemisinin, menthol, and saponins—exhibiting antimalarial, antimicrobial, and lipid-lowering activities [21].
Alkaloids, nitrogen-containing molecules like morphine, caffeine, and berberine, occur predominantly in medicinal plants and are well-documented for pharmacological activities in traditional and modern medicine [22]. Glucosinolates, characteristic of Brassicaceae vegetables, degrade into biologically active isothiocyanates—potent agents for chemoprevention through epigenetic modulation and detoxification enzyme activation [23].
Despite compelling in vitro and in vivo preclinical data, the translational potential of phytochemicals is frequently constrained by issues of solubility, absorption, metabolism, and bioavailability. Strategies to overcome these limitations include nanoformulation, prodrugs, complexation with cyclodextrins, and co-administration with absorption enhancers. Phytochemicals have shown efficacy in the chemoprevention and adjunct treatment of various cancers, neurodegenerative diseases, and metabolic disorders.
4. Common Allopathic Treatments for Neurological Diseases
Allopathic medicine combines neurological diseases with pharmaceutical drugs and specialized therapies. These treatments are for managing symptoms, slowing disease progression, and improving quality of life.
4.1 Alzheimer's Disease
Alzheimer’s disease (AD) is managed using a spectrum of pharmacological agents aimed at alleviating symptoms and modifying disease progression. The various classes of allopathic drugs used are illustrated in Figure 2.
Cholinesterase inhibitors (ChEIs) such as donepezil and galantamine are commonly prescribed to enhance cholinergic neurotransmission by inhibiting acetylcholinesterase (AChE), thereby increasing acetylcholine levels in synapses. Galantamine, notably derived from Amaryllidaceae plants, also exhibits nicotinic receptor allosteric modulation and contains bioactive alkaloids that may contribute to its efficacy [24,25].
The NMDA receptor antagonist memantine is another cornerstone treatment, particularly for moderate-to-severe AD. Memantine modulates glutamatergic activity by antagonizing NMDA receptors, thereby preventing excitotoxic neuronal damage caused by excessive glutamate—a hallmark in AD pathology. It is well-tolerated and often combined with donepezil to target both cholinergic and glutamatergic dysfunctions [26,27].
In terms of disease-modifying therapies (DMTs), the recent introduction of anti-amyloid monoclonal antibodies represents a significant advancement. Aducanumab, a monoclonal antibody targeting aggregated amyloid-β (Aβ42), preferentially binds to fibrillar deposits and was the first FDA-approved DMT in June 2021 for early-stage AD patients with confirmed amyloid pathology. Despite controversies regarding its clinical benefit and approval process, aducanumab marked a paradigm shift in AD management by targeting pathophysiological mechanisms rather than symptoms alone [28,29].
Lecanemab, another FDA-approved monoclonal antibody, selectively binds to soluble Aβ protofibrils. It was derived from the mouse mAb158 and received accelerated FDA approval in January 2023, followed by full approval in July 2023. Lecanemab is indicated for patients with mild cognitive impairment or mild AD dementia and confirmed amyloid pathology. Its biweekly intravenous regimen and well-defined targeting profile make it a promising DMT option, although concerns remain about amyloid-related imaging abnormalities (ARIA) and long-term safety [30].
Overall, while agents like donepezil, galantamine, and memantine offer symptomatic relief, monoclonal antibodies such as aducanumab and lecanemab represent an emerging strategy focused on modifying disease progression. However, clinical application necessitates early diagnosis, amyloid positivity confirmation, and monitoring for adverse effects—highlighting the evolving landscape of Alzheimer’s disease therapeutics [31,32].
4.2 Parkinson's Disease
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra. The various classes of allopathic drugs used for PD are illustrated in Figure 3.
The first-line treatment remains levodopa, often administered with carbidopa to inhibit peripheral metabolism and enhance central nervous system delivery. Levodopa acts as a dopamine precursor and significantly improves motor symptoms such as bradykinesia, rigidity, and tremor. Although generally well tolerated, levodopa-carbidopa therapy may induce long-term side effects including dyskinesias, hallucinations, and gastrointestinal disturbances [33].
In early or fluctuating stages of PD, dopamine agonists such as pramipexole and ropinirole are often employed. These agents selectively activate D2-like dopamine receptors and can delay the initiation of levodopa therapy. Pramipexole and ropinirole have demonstrated significant efficacy in reducing motor symptoms and improving quality of life, although they are associated with adverse effects such as impulse control disorders, orthostatic hypotension, and sleep disturbances [34,35].
Another key pharmacological approach includes monoamine oxidase-B (MAO-B) inhibitors, specifically rasagiline and selegiline. These agents inhibit the enzymatic degradation of dopamine, thereby prolonging its synaptic availability. Rasagiline, in particular, has been shown to exert greater potency than selegiline and has demonstrated disease-modifying potential in early PD when used at 1 mg/day [36].
Overall, pharmacologic management of PD requires a tailored approach that evolves with disease progression. Combination therapies, such as dopamine agonists with levodopa or adjunctive MAO-B inhibitors, are commonly employed to mitigate motor complications and enhance therapeutic efficacy.
4.3 Epilepsy
The management of epilepsy primarily involves long-term administration of antiepileptic drugs (AEDs) aimed at stabilizing neuronal excitability and preventing seizure recurrence. The various classes of allopathic drugs used for epilepsy are illustrated in Figure 4.
A major class of AEDs—sodium channel blockers—includes phenytoin and lamotrigine, which stabilize neuronal membranes by promoting the fast inactivation of voltage-gated sodium channels. Newer agents, such as lacosamide and eslicarbazepine acetate, act on the slow inactivation phase of sodium channels, potentially improving tolerability and reducing cognitive side effects at therapeutic doses [37,38].
Older drugs like phenytoin and carbamazepine remain effective but are often limited by enzyme induction and complex pharmacokinetics, prompting a shift toward newer agents with favorable safety profiles [39].
Modulstors of gamma amino butyric acid (GABA), including valproic acid and phenobarbital, exert their antiepileptic effect by enhancing GABAergic inhibition. These compounds increase chloride influx into neurons via GABA-A receptor activation, producing hyperpolarization and reducing neuronal excitability. Additional agents such as tiagabine (a GABA reuptake inhibitor) and vigabatrin (an irreversible inhibitor of GABA transaminase) offer alternative means to elevate synaptic GABA levels, contributing to seizure control—particularly in refractory or infantile epilepsy settings [40,41].
Another mechanistically distinct class includes modulators of synaptic vesicle glycoprotein 2A (SV2A), typified by levetiracetam, which bind to the SV2A. This membrane protein regulates calcium-dependent neurotransmitter release, especially of GABA and glutamate [42]. SV2A dysfunction has been implicated in epileptogenesis and developmental epileptic encephalopathies. Levetiracetam’s precise binding to SV2A facilitates broad-spectrum seizure control with minimal drug-drug interactions, making it a preferred option for both monotherapy and adjunctive use [43,44].
4.4 Spinal Cord-Related Diseases
Treatment of spinal cord-related disorders primarily targets inflammation, spasticity, and neuropathic pain to improve function and quality of life. The various classes of allopathic drugs used for spinal cord injury are illustrated in Figure 4. In acute spinal cord injury (SCI), high-dose methylprednisolone has historically been used to reduce inflammation and limit secondary neuronal damage, although its routine use is now controversial due to mixed outcomes and adverse effects [45]. Muscle relaxants, especially GABA-B receptor agonists like baclofen and tizanidine, are frequently used to manage spasticity associated with spinal cord injury or multiple sclerosis (MS). These drugs act centrally to inhibit excitatory neurotransmission at the spinal level, effectively reducing muscle tone and spasm frequency [46,47].
For neuropathic pain, particularly post-SCI, gabapentin and pregabalin—structural analogs of GABA—are frontline agents. They bind to the α2δ subunit of voltage-gated calcium channels, inhibiting excitatory neurotransmitter release. These agents are effective in managing pain and paresthesia, though they require dose titration to balance efficacy with side effects such as dizziness or sedation [48]. A distinct and debilitating complication, complex regional pain syndrome (CRPS), often arises after peripheral nerve trauma and is characterized by central sensitization, autonomic dysregulation, and neuroimmune inflammation. Dysregulated C-fiber signaling and increased adrenergic receptor expression contribute to symptoms such as allodynia and hyperalgesia [49]. Pharmacologic interventions in CRPS may include ketamine infusions (an NMDA receptor antagonist), bisphosphonates, low-dose naltrexone (an opioid receptor modulator with neuroinflammatory effects), and botulinum toxin A for regional pain relief. Ketamine has shown efficacy even in later stages of CRPS, while naltrexone's immunomodulatory properties are gaining recognition in chronic pain management [50,51]. Ultimately, optimal outcomes require multidisciplinary approaches that integrate pharmacotherapy with physical rehabilitation, psychosocial support, and interventional techniques to mitigate the long-term burden of spinal cord-related diseases.
4.5 Traumatic Brain Injury
Traumatic brain injury (TBI) is a leading neurological disorder resulting from external mechanical forces such as impact, acceleration, or blast injuries [52]. It presents a spectrum of clinical manifestations, ranging from mild concussion to severe brain damage, and is associated with substantial morbidity and mortality globally. Allopathic treatment strategies for TBI are multifaceted and aim to prevent secondary injury, promote neuroprotection, and manage long-term cognitive, motor, and psychiatric sequelae (Figure 5). In the acute phase, the priority is stabilization of vital functions using Advanced Trauma Life Support (ATLS) protocols. Airway protection, ventilation, and hemodynamic stabilization are critical. Intracranial pressure (ICP) management is central to initial care, with hyperosmolar agents such as mannitol and hypertonic saline commonly used to prevent cerebral herniation and further neuronal injury. These agents reduce cerebral edema and improve perfusion pressure, a determinant of outcomes in severe TBI cases [53].
Sedation and analgesia using agents such as propofol and midazolam reduce cerebral metabolic demand and prevent agitation, which can worsen intracranial hypertension. In cases of refractory ICP elevation, therapeutic options include induced barbiturate coma and decompressive craniectomy. Antiepileptic drugs (AEDs), especially levetiracetam and phenytoin, are often administered prophylactically in the first week post-injury to reduce early post-traumatic seizures, although their role in preventing long-term epilepsy remains inconclusive [54]. Pharmacological neuroprotection has received increasing attention, particularly in the subacute and chronic phases of TBI (Figure 5). Agents such as amantadine, an NMDA receptor antagonist with dopaminergic activity, have shown benefits in enhancing recovery of consciousness and functional outcomes in moderate to severe TBI [55]. Methylphenidate, commonly used in attention-deficit disorders, is employed to improve attention span and cognitive processing speed in TBI patients experiencing cognitive fatigue [56]. Similarly, melatonin has been studied for its antioxidant and anti-inflammatory properties in reducing neuronal damage and enhancing post-injury neurocognitive function [57].
The inflammatory cascade following TBI, characterized by microglial activation, cytokine release, and disruption of the blood-brain barrier, presents another therapeutic target. While corticosteroids such as methylprednisolone were historically used to blunt neuroinflammation, large-scale trials like CRASH have revealed increased mortality associated with their use, leading to a shift away from corticosteroid-based therapies. Research is now focused on specific cytokine inhibitors and biologics that modulate immune responses without systemic immunosuppression [58,59]. Cognitive and neuropsychiatric symptoms following TBI—including depression, emotional dysregulation, agitation, and anxiety—are often managed with selective serotonin reuptake inhibitors (SSRIs) such as sertraline and escitalopram [60,61].
Recent research has explored novel pharmacologic agents for TBI management. Statins have shown anti-inflammatory and endothelial stabilizing effects in animal models, with early-phase human trials suggesting potential benefits in reducing cerebral edema [62]. Progesterone and erythropoietin, though promising in preclinical studies for neuroprotection and myelin repair, have failed to demonstrate efficacy in large randomized controlled trials [63]. Cannabinoids, particularly cannabidiol (CBD), are being investigated for their potential to reduce anxiety, neuroinflammation, and seizure risk, although robust clinical data in TBI populations remain scarce [64].
In post-acute and chronic stages, pharmacotherapy is complemented by comprehensive neurorehabilitation. Botulinum toxin A injections are used to manage focal spasticity, while oral muscle relaxants like baclofen and tizanidine alleviate central hypertonicity. Beta-blockers and clonidine may be used to control paroxysmal sympathetic hyperactivity, a syndrome of autonomic instability seen in some severe TBI cases. In summary, the allopathic approach to TBI incorporates a range of evidence-based interventions aimed at stabilizing acute injury, reducing secondary damage, and managing long-term complications. While no single pharmacologic agent has demonstrated universal efficacy in reversing TBI pathology, a symptom-targeted, multimodal strategy integrating pharmacological and rehabilitative interventions remains the current standard. Ongoing research into inflammatory modulators, neurostimulants, and regenerative therapies may further enhance recovery and quality of life in individuals living with TBI.
5. Phytochemicals That May Help in Neurological Diseases
Phytochemicals—bioactive compounds derived from plants—have gained considerable attention for their potential therapeutic effects in neurological disorders. Many of these natural compounds exhibit antioxidant, anti-inflammatory, neuroprotective, and cognitive-enhancing properties. As conventional treatments often focus on symptom management, phytochemicals offer promising complementary or alternative strategies for targeting the underlying mechanisms of neurological diseases (Figure 6).
5.1 Alzheimer’s disease
Phytochemicals—naturally occurring plant-based compounds—have emerged as promising multi-target agents in the prevention and management of AD. Unlike synthetic drugs that largely provide symptomatic relief, phytochemicals exert disease-modifying effects by acting on key pathological hallmarks such as amyloid-β (Aβ) aggregation, tau hyperphosphorylation, neuroinflammation, oxidative stress, mitochondrial dysfunction, and synaptic loss. These compounds are chemically diverse and can be broadly categorized into polyphenols, alkaloids, terpenoids, and volatile phytochemicals.
5.1.1 Polyphenols
Resveratrol, a stilbene polyphenol found in grapes and red wine, exhibits antioxidant, anti-inflammatory, and anti-amyloid effects by modulating signaling pathways involved in Aβ aggregation and tau phosphorylation. A phase II clinical trial confirmed its efficacy in reducing cerebrospinal fluid biomarkers of neuronal injury and inflammation in AD patients [65,66]. Its analog polydatin, with superior bioavailability, showed cognitive and biochemical improvements in aluminum chloride-induced AD rat models [67].
To improve delivery, chitosan-coated bovine serum albumin nanoparticles (CS-RES-BSANPs) were developed for intranasal administration, demonstrating enhanced brain penetration and behavioral outcomes [68]. Similarly, dihydro-resveratrol (DHR), a microbial metabolite, improved cognition in AD mouse models by enhancing mitophagy via the ULK1-Bnip3 pathway [69].
Another key polyphenol, Curcumin, the main bioactive compound in Curcuma longa (turmeric), has demonstrated potent antioxidant, anti-inflammatory, anti-amyloid (Aβ), and anti-tau properties in Alzheimer’s disease (AD) models [70]. To address its low solubility and poor bioavailability, several nanocarrier-based delivery systems have been developed. These include MWCNT-COOH-PEG, which enhances brain targeting and provides sustained release [71]. curcumin-silver nanoparticles with notable acetylcholinesterase inhibition and antioxidant activity [72]. BP-PEG-Tar@Cur, which reduces Aβ aggregation and oxidative stress in AD mice [73]. C3/TPP-EXO-CUR, an engineered exosome system targeting damaged mitochondria to lower tau phosphorylation and neuronal apoptosis [74], and EXO-Cur+MB, a co-delivery exosomal system with methylene blue that effectively inhibits tau hyperphosphorylation via the AKT/GSK-3β pathway [75].
Further strategies included curcumin-enriched mesenchymal stem cell exosomes (CUR-MSC-EXO), which modulated microglial polarization and improved memory [76], and curcumin-selenium nanoemulsions, which reduced oxidative stress and tau/Aβ burden [77]. Diagnostic approaches using [18F]-DiFboron-8, a PET imaging probe derived from half-curcumin, enabled non-invasive Aβ plaque detection [78], while ophthalmoscopy using curcumin contrast identified retinal Aβ deposits, aiding early AD diagnosis [79]. A chemically modified curcumin derivative, Derivative 27, activated the Nrf2 pathway and significantly reduced hippocampal Aβ burden and inflammation, demonstrating enhanced efficacy at lower doses [80].
Quercetin, a flavonoid abundant in apples, onions, and leafy greens, exerts neuroprotective effects through oxidative stress inhibition, tau dephosphorylation, and anti-inflammatory activity. Encapsulated as nanostructured lipid carriers (QC-NLCs), quercetin improved brain uptake and decreased Aβ deposition in rat models [81]. It also inhibited early stress-induced AD pathology via NLRP3 inflammasome modulation [82]. and acted synergistically with donepezil to reduce tau and BACE1 expression while restoring miRNA-124 levels [83]. Additionally, quercetin modulated P2X7 receptors, implicated in ATP-mediated neuroinflammation [84]. To enhance gut delivery and systemic efficacy, quercetin-decorated selenium nanoparticles (QUE@SeNPs) within chitosan/PVP nanofibers were developed. This system improved gut–liver–brain axis regulation and cognitive outcomes through microbiota modulation and gut barrier restoration [85].
Another promising polyphenol, Dip-1 from Dipteris wallichii, was identified as a potent BACE1 inhibitor, exhibiting high BBB permeability and a low IC50 value, supporting its role in Aβ pathology suppression [86].
5.1.2 Alkaloids
Berberine (BBR), an isoquinoline alkaloid extracted from Berberis vulgaris, offers neuroprotection through dual mechanisms: central anti-inflammatory and anti-amyloid effects, and peripheral modulation of the gut–brain axis. In AD models, it reduced Aβ plaques, enhanced spatial memory, and improved gut microbiota diversity and barrier function [87]. Importantly, berberine reversed D-ribose-induced cognitive impairment by demethylating the PINK1 promoter, restoring mitophagy via the PINK1–Parkin pathway [88]. A meta-analysis of 19 animal studies confirmed its efficacy in improving cognition and downregulating APP expression [89].
5.1.3 Terpenoids
Ginsenoside Rb1, a triterpenoid saponin from Panax ginseng, has shown therapeutic efficacy in reducing Aβ burden, tau hyperphosphorylation, oxidative stress, and neuronal apoptosis. It modulates apoptotic signaling by decreasing Bax and cleaved caspase-3 while increasing Bcl-2 expression, leading to improved cognitive and memory functions in AD models [90,91]. Ginkgolide, a diterpenoid lactone from Ginkgo biloba, demonstrated anti-inflammatory and anti-amyloid properties. In APP/PS1 mice, it attenuated Aβ accumulation, reduced activation of the NLRP3 inflammasome, and lowered pro-inflammatory cytokines such as IL-1β and ROS, underscoring its potential in halting AD-associated neuroinflammation [92].
5.1.4. Volatile Phytochemicals and Lignans
Several volatile compounds exhibit anti-amyloid and anti-tau actions. Cinnamaldehyde, phenylethyl alcohol, α-asarone (ASA), and β-caryophyllene (BCP), sourced from aromatic plants, prevented Aβ(25–35) fibrillation and reduced β-sheet content, as confirmed by spectroscopic and microscopic analyses [93]. ASA and BCP also disassembled pre-formed tau fibrils and protected neuroblastoma cells against tau-induced toxicity [94].
Among lignans, secoisolariciresinol diglucoside (SDG) from flaxseed demonstrated cognitive benefits in transgenic female AD mice. SDG suppressed Aβ deposition and neuroinflammation, enhanced GPER-CREB/BDNF signaling, and modulated gut microbiota, linking its action to gut-brain communication [95].
5.2 Parkinson’s Related Phytochemicals
These compounds target various pathological mechanisms of PD including oxidative stress, mitochondrial dysfunction, α-synuclein aggregation, and neuroinflammation. This section outlines key classes of phytochemicals that show promise in PD management, focusing on specific compounds and their mechanisms of action.
5.2.1 Flavonoids
Flavonoids, a major class of phytochemicals, exhibit neuroprotective effects primarily through antioxidant and anti-inflammatory mechanisms. Baicalein, derived from Scutellaria baicalensis (Chinese skullcap), targets apoptotic and oxidative stress-related pathways. It interacts with key proteins such as MAPK1, EP300, and CREBBP [96], and in a 6-hydroxydopamine (6-OHDA) PD model, activates mitochondrial autophagy via the miR-30b-5p/SIRT1/AMPK/mTOR axis, reducing neuronal apoptosis [97]. Its metabolite baicalin also exerts neuroprotection by activating Nrf2 and suppressing NLRP3 inflammasome signaling in both in vitro (α-syn/MPP﹢) and in vivo (MPTP) PD models [98].
Epigallocatechin gallate (EGCG), a catechin from green tea, protects dopaminergic neurons through antioxidant and mitochondrial-stabilizing effects [99]. In 6-OHDA-induced SK-N-AS cells, it enhances viability, inhibits caspase-3, and reduces IL-1β [100]. Liposomal EGCG formulations (PC/PS-based, vitamin E-coated) improved uptake and suppressed LPS-induced neuroinflammation in PD rats [101], while in rotenone-induced models, EGCG preserved neurotransmitter levels and mitochondrial integrity [102].
Quercetin, found in apples, onions, and cocoa husk (GuaCa extract), alleviates motor and non-motor symptoms in PD. It reduces hippocampal IL-6, preserves dopaminergic neurons, enhances BDNF, and activates PI3K/Akt/GSK-3β signaling [103-105]. Nanoformulations, such as QAE NPs and quercetin-loaded niosomes, facilitate intranasal delivery, improving mitochondrial homeostasis and M2 microglial polarization [106,107]. Additionally, quercetin inhibits NLRP3 inflammasome activation in vitro (e.g., from Cola acuminata) [108].
Apigenin, from Campsis grandiflora, promotes chaperone-mediated autophagy, clears α-synuclein aggregates, and activates Nrf2. It modulates PI3K/Akt/NF-κB pathways and reduces caspase-3 activity [109,110]. In MPTP-induced mice, apigenin normalized cytokine profiles and ameliorated histological damage [111]. Docking studies confirm its binding affinity to PD-relevant targets including α-synuclein [112].
Rutin, a glycoside flavonoid found in Berula erecta and other sources, improves motor and gastrointestinal functions via modulation of enteric glial reactivity and nitric oxide signaling [113]. It also restores neurotransmitter balance and cognitive function in rotenone-induced models [114].
5.2.2 Polyphenols and Stilbenes
Resveratrol, a stilbene from grapes and berries, enhances lifespan, motor function, and oxidative balance in transgenic Drosophila expressing α-synuclein [115]. In MPP﹢-treated SH-SY5Y cells, resveratrol-loaded neural stem cell-derived exosomes (RES-hNSCs-Exos) restored mitochondrial function and suppressed NLRP3 via AMPK-Nrf2 activation [116]. Leptin/transferrin-decorated nanoparticles co-loaded with resveratrol and ceftriaxone targeted dopaminergic neurons and modulated pathways like MAPK/ERK and α-synuclein [117]. Solid lipid nanoparticle microneedle patches improved resveratrol bioavailability, antioxidant capacity, and behavior without skin irritation [118]. In A53T PD mice, resveratrol restored mitochondrial VDAC1 function, reducing α-synuclein–VDAC1 interaction and calcium imbalance [119].
Oligomeric stilbenes (OSs) from Alpha grape stems (e.g., vitisin A, trans-vitisin B) showed antioxidant neuroprotection in vitro [120], while α-viniferin, a resveratrol trimer, exhibited potent MAO inhibition, dopamine enhancement, and motor improvement without toxicity in Drosophila and murine models [121].
5.2.3 Alkaloids
Alkaloids, a diverse class of nitrogen-containing phytochemicals, have shown promising neuroprotective potential in Parkinson’s disease (PD) by modulating neurotransmission, inhibiting neuroinflammation, and supporting neuroplasticity. Harmine and harmaline, alkaloids from Peganum harmala, act as MAO-A inhibitors, increasing serotonin, dopamine, and noradrenaline levels. They also activate Nrf2, suppress NF-κB, and enhance BDNF/TrkB, improving PD-related behavioral and oxidative parameters in stress-induced rats [122].
Humulus japonicus water extract (HJW) countered scopolamine-induced cognitive decline by inhibiting acetylcholinesterase and activating CaMKIIα-CREB and AKT-GSK3β pathways, supporting neuroplasticity [123]. Although synthetic, PZKKN-94, a 5-HT1B agonist/5-HT6 antagonist, mimics phytochemical serotonergic activity seen in agents like resveratrol and curcumin, providing neuroprotection, antidepressant-like effects, and cognitive enhancement in PD models [124]. A blend of safflower seeds (Carthamus tinctorius) and dandelion (Taraxacum coreanum) extracts (CTS-TC) improved cognition and reduced neuroinflammation in scopolamine-treated models. HPLC identified active constituents including N-feruloylserotonin, chicoric acid, and chlorogenic acid with antioxidant and neuromodulatory properties [125].
5.2.4 Phenolic Acids
Phenolic acids, especially hydroxycinnamic acids, target α-synuclein aggregation and neuroinflammation. Sinapic acid and chlorogenic acid inhibit α-synuclein fibrillation and suppress its β-sheet conversion, even post-aggregation, demonstrating utility at various PD stages [126]. Ferulic acid (FA), found in grains and fruits, improves dopaminergic survival, mitochondrial gene expression, TH levels, and reduces α-synuclein, oxidative stress, and NF-κB activity in MPTP and rotenone-induced models [127,128]. Caffeic acid, abundant in Brassica juncea and Eucommia ulmoides, preserves dopaminergic neurons and regulates autophagy (e.g., LC3b, ATG7, α-syn) through 4E-BP1 activation [129]. Embedded in hydrogels like OACDP, it offers controlled delivery and mitigates neuroinflammation and oxidative injury [130]. Caffeic acid-derived carbon quantum dots (CACQDs) protect against paraquat-induced toxicity via free radical scavenging and anti-aggregation properties [131]. Synthetic phenolic derivatives based on proline and GABA scaffolds show multitarget effects, including lipid peroxidation inhibition, anti-inflammatory activity, and mild acetylcholinesterase inhibition—relevant to PD pathology [131].
5.3 Epilepsy-Related Phytochemicals
Phytochemicals offer promising alternatives for managing epilepsy, particularly drug-resistant forms, through diverse mechanisms including modulation of neurotransmission, anti-inflammatory activity, antioxidant defense, and gut-brain axis regulation. These compounds are broadly classified into cannabinoids, monoterpenes, alkaloids, flavonoids, polyphenols, and saponins.
5.3.1 Cannabinoids
Cannabidiol (CBD), a non-psychoactive compound from Cannabis sativa, is FDA- and EMA-approved for seizures in tuberous sclerosis and under investigation for broader neuropsychiatric symptoms [133]. Multiple preclinical studies reinforce CBD’s anticonvulsant efficacy. Preclinical models (e.g., zebrafish, SE) show its anticonvulsant effects and glutamate modulation [134,135]. Clinically, CBD significantly reduces seizure frequency in drug-resistant epilepsy (DRE), with up to 50% responder rates, though higher doses may cause side effects [136-138]. Mechanistically, CBD suppresses reactive astrocytes and neuroinflammation, while pharmacokinetic studies highlight interindividual variability and the need for liver function monitoring [139,140]. Therapeutic drug monitoring (TDM) studies reveal significant pharmacokinetic variability in CBD and its metabolite 7-hydroxy-CBD, emphasizing the need for individualized dosing and liver function monitoring due to moderate ALT elevations [140]. A review of 47 clinical trials confirmed CBD’s anticonvulsant effects, particularly in Dravet and Lennox-Gastaut syndromes, while stressing the need for standardized dosing and trial protocols [141]. Additionally, non-cannabis analogs such as Magnolia spp.-derived magnolol and honokiol, and amorfrutin 2 from Amorpha fruticosa, inhibit T-type calcium channels, offering cannabinoid-independent anticonvulsant activity [142].
5.3.2 Monoterpenes
Monoterpenes, volatile compounds primarily found in essential oils, exhibit strong anticonvulsant and neuroprotective effects. (+)-Borneol, from Dryobalanops aromatica, enhanced the potency and brain levels of retigabine, significantly reducing required doses in resistant seizure models [143]. Geraniol, present in Cymbopogon and Pelargonium, provided protection via GABAergic enhancement, oxidative stress reduction, and cytokine modulation [144]. Alpha-pinene, a major constituent of Pinus species, attenuated seizures in kainic acid models by downregulating NF-κB and ERK1/2 signaling and reducing glial activation [145].
5.3.3 Alkaloids
Alkaloids are nitrogen-containing compounds known for potent neuroactivity. Stachydrine, from Leonurus japonicus, showed dual inhibition of Notch1 and NMDA receptor pathways in PTZ-induced mice, reducing excitotoxicity, inflammation, and cognitive decline [146]. Berberine (BBR), from Berberis vulgaris, alleviated hippocampal damage and modulated the gut-brain axis by increasing beneficial microbiota and altering SCFA and brain lipid profiles, highlighting microbiome-mediated anticonvulsant pathways [147].
5.3.4 Flavonoids
Flavonoids, abundant in fruits and herbs, act via antioxidant, anti-inflammatory, and GABAergic pathways. Quercetin, found in onions and apples, reduced seizure duration and preserved antioxidant enzymes when delivered via chitosan nanoparticles [148]. Extracts from Cyanthillium cinereum, rich in quercetin, kaempferol, and gallic acid, delayed seizure onset and improved oxidative status [149].
Luteolin, from Salvia miltiorrhiza, inhibited apoptosis and inflammation through MAPK/NF-κB and GADD45B targeting in kainic acid models [150]. Glabranin and 3'-hydroxy-4'-O-methylglabridin from Glycyrrhiza glabra were identified via AI-guided modeling as AKT1 inhibitors [151]. Kaempferol, from tea and broccoli, showed high brain delivery via intranasal phospholipid magnesomes and sustained anti-seizure effects [152]. Apigenin, present in parsley and chamomile, raised seizure thresholds by increasing GABA-A receptor expression [153]. Naringin, a citrus flavonoid, reduced seizures and cognitive deficits by suppressing HMGB1-TLR4 signaling and enhancing neuroprotective factors like Klotho and ADAM-10 [154].
5.3.5 Polyphenols
Polyphenols offer antioxidant and anti-inflammatory benefits in seizure control. Caffeic acid, abundant in coffee, reduced seizures and neuroinflammation by inhibiting the PERK–NF-κB pathway via aconitate decarboxylase 1 binding [155]. In Origanum majorana, caffeic acid and quercetin delayed seizures through NMDA receptor interaction [156]. Curcumin and its analog mitocurcumin (MitoCur) reduced oxidative stress in zebrafish seizure models, with MitoCur displaying higher efficacy at low doses but pro-oxidant effects at higher levels [157]. Wogonin, from Scutellaria baicalensis, restored synaptic density and suppressed microglial overactivation via AKT/FoxO1 signaling [158]. Lippia multiflora, rich in polyphenols and flavonoids, improved seizure outcomes and normalized TNF-α, IL-1β, and IL-6 levels in chronic epilepsy models [159].
5.3.6 Saponins
Saponins, particularly steroidal and triterpenoid forms derived from various medicinal plants, have shown significant antiepileptic potential by modulating neuronal excitability, neurotransmission, mitochondrial function, and inflammation.
From Solanum torvum, steroidal saponins like torvosides A, X, Y showed strong activity in zebrafish via glycosylation at C-6 [160]. Saponins from Anemarrhena asphodeloides (AAS) mitigated hippocampal neuron loss and downregulated epilepsy-related proteins HSP90AB1 and YWHAB [161]. Ginsenoside Re (GRe) from Panax ginseng reduced mitochondrial oxidative stress and seizures, partly via IL-6/STAT3 signaling [162]. Diosgenin, from Dioscorea spp., improved gut microbiota, reduced glial activation, and modulated TLR4-MyD88 signaling, linking its effect to gut–brain axis regulation [163]. Saikosaponin-a (SSa), from Bupleurum spp., encapsulated in MePEG-PCL nanoparticles, enhanced brain delivery and significantly reduced seizures, neuronal apoptosis, and hippocampal damage while increasing GABA-A receptor levels [164].
5.4 Spinal Cord-Related Phytochemicals
SCI is characterized by an initial mechanical insult followed by a cascade of secondary injuries such as inflammation, oxidative stress, apoptosis, and autophagy, which worsen neuronal damage. Certain phytochemicals have demonstrated neuroprotective effects by modulating these secondary injury pathways.
5.4.1 Flavonoids
Flavonoids are plant-derived polyphenolic compounds known for their potent antioxidant, anti-inflammatory, and neuroprotective effects. Several studies have highlighted their therapeutic potential in managing spinal cord injury (SCI) and related neuropathies.
Corn silk extract (CSE) from Zea mays stigmas contains flavonoids such as maysin, apigenin, and luteolin. It demonstrated neuroprotective effects in paclitaxel-induced peripheral neuropathy (PIPN) by reducing oxidative stress, inflammation, and overactivation of the renin–angiotensin system. CSE preserved spinal cord and sciatic nerve structure, reduced demyelination, and downregulated NF-κB and ACE activity, supporting its use in multi-targeted neuropathic treatment [165].
Catechin, found in green tea (Camellia sinensis), cocoa, and fruits, showed efficacy in a chronic constriction injury model by reducing mechanical hyperalgesia and suppressing neuroinflammation. It inhibited iNOS, COX-2, and pro-inflammatory cytokines, while promoting an anti-inflammatory M2 microglial phenotype via suppression of the TLR4/MyD88/NF-κB and MAPK pathways [166].
Calycosin, an isoflavone from Astragalus membranaceus, mitigated glial scar formation in SCI by inhibiting A1 astrocyte activation. It reduced complement C3 expression and STAT3 phosphorylation, suggesting its regulatory effect on astrocyte-mediated inflammation [167].
Quercetin, widely present in Allium cepa, Malus domestica, and Camellia sinensis, inhibited ferroptosis in oligodendrocyte progenitor cells by blocking NCOA4-mediated ferritinophagy. It preserved mitochondrial integrity, reduced lipid peroxidation, and maintained iron homeostasis, indicating its protective role in SCI [168]. Quercetin also showed antinociceptive effects in a spinal cord hemi-contusion model when derived from Trifolium resupinatum essential oil, through modulation of NO-cGMP-K﹢pathways and TRPV1/CB1 receptor interactions, along with suppression of inflammatory cytokines [169].
Baicalin, extracted from Scutellaria baicalensis, exerted anti-inflammatory effects in SCI by binding the TLR4/MD2 complex on microglia and inhibiting PI3K/AKT/NF-κB signaling. It reduced glial activation and indirectly suppressed astrocyte-mediated inflammation by decreasing TNF-α, IL-1α, and C1q levels [170].
Rutin, present in Fagopyrum esculentum, apples, and citrus fruits, improved locomotor recovery and reduced neuroinflammation in a distraction spinal cord injury model. It inhibited the P38 MAPK/NF-κB/STAT3 axis and attenuated neuronal apoptosis and demyelination. Molecular docking confirmed its interaction with MAPK13 [171].
Rosmarinic acid, a polyphenolic compound from Rosmarinus officinalis, Ocimum basilicum, and Origanum vulgare, promoted sciatic nerve regeneration after taxol-induced injury. It inhibited NF-κB signaling, enhanced CGRP expression and axon number, although behavioral outcomes remained unchanged [172].
- Alkaloids
Alkaloids, nitrogen-containing secondary metabolites commonly derived from medicinal plants, have demonstrated significant neuroprotective potential in spinal cord injury (SCI) through their antioxidant, anti-inflammatory, and anti-ferroptotic properties.
Aloperine, a quinolizidine alkaloid from Sophora spp., improved locomotor function and preserved spinal tissue in a rat contusion SCI model. It reduced markers of apoptosis (Bax, cleaved caspase-3), oxidative stress (4HNE, MDA), and inflammation (NF-κB, TNF-α), while upregulating antioxidant enzymes (SOD1, GPx1) through activation of PI3K/AKT and inhibition of NF-κB pathways [173].
Berbamine, extracted from Stephania epigaea, alleviated neuropathic pain in a chronic constriction injury model by inhibiting the TMEM34/SGK1/FOXO3 axis, thus reducing astrocyte and neuron activation and behavioral signs of allodynia and hyperalgesia [174]. Tetrahydroberberine (THB), derived from Corydalis spp. (family Papaveraceae), significantly improved motor recovery and tissue repair in SCI mice by activating the Nrf2 pathway, reducing lipid peroxidation, and inhibiting ferroptosis. The protective effects were negated upon Nrf2 inhibition, confirming pathway involvement [175].
Tomatidine, a steroidal alkaloid from Solanum lycopersicum (tomato), promoted neuronal recovery, reduced apoptosis and inflammation in vivo and in vitro, and exerted its effects by downregulating CXCL10 and inhibiting the NF-κB pathway [176].
Tetrandrine, a bis-benzylisoquinoline alkaloid from Stephania tetrandra, showed neuroinflammatory modulation in SCI therapy. Delivered via MPEG-PDLLA nanoparticles embedded in GelMA microgels, it suppressed neurotoxic glial crosstalk and enhanced spinal repair post-injection [177].
Tetramethylpyrazine (TMP), isolated from Ligusticum chuanxiong, improved motor function and reduced neuronal death in SCI by inhibiting ferroptosis. TMP activated the NRF2/ARE pathway to regulate antioxidant gene expression and maintain iron homeostasis [178].
Berberine (BBR), an isoquinoline alkaloid from Berberis vulgaris, mitigated SCI-induced ferroptosis by reducing lipid peroxidation, iron accumulation, and mitochondrial dysfunction. It activated the AMPK-NRF2-HO-1 pathway, and inhibition of AMPK reversed its protective effects, confirming mechanistic specificity [179].
5.4.3 Polyphenols
Polyphenols, widely distributed in plants, possess strong antioxidant, anti-inflammatory, and neuroprotective properties, making them valuable therapeutic agents in spinal cord injury (SCI).
Epigallocatechin gallate (EGCG), a major catechin derived from Camellia sinensis (green tea), has demonstrated neuroregenerative effects in SCI models. EGCG reduced oxidative stress by activating the Keap1/Nrf2/HO-1 pathway and upregulating antioxidant enzymes such as SOD1 and SOD2. Incorporated into stem cell sheets or fibrin scaffolds, EGCG enhanced cell viability, promoted angiogenesis and axonal regeneration, and improved functional outcomes in rats with SCI [180,181].
Resveratrol, a stilbene polyphenol found in Vitis vinifera (grape), Arachis hypogaea (peanut), and various berries, has been extensively studied for SCI therapy. In exosome-based delivery systems, resveratrol targeted inflamed spinal tissues, inhibited reactive oxygen species (ROS), reduced glial scarring, and modulated A1 astrocyte activity [182]. Resveratrol also reduced pyroptosis by upregulating miR-124-3p and downregulating DAPK1, thereby inhibiting the NLRP3/caspase-1/GSDMD pathway [183]. Additionally, it improved the anti-inflammatory effects and survival of bone marrow mesenchymal stem cells (BM-MSCs) by activating SIRT1 and suppressing NF-κB, resulting in enhanced neuroprotection and motor recovery [184]. Combined with calcium, resveratrol further showed promise in preventing SCI-induced osteoporosis through SIRT1/FOXO3a signaling, improving bone microarchitecture and resistance to fracture [185]. Moreover, polyphenolic extracts from grape stalks and Coffea arabica (coffee) reduced neuropathic pain and modulated neuroimmune pathways (CCL2/CCR2, CX3CL1/CX3CR1) in supraspinal regions, reducing glial activation and behavioral hypersensitivity in mice [186].
Curcumin, a polyphenol from Curcuma longa (turmeric), has shown diverse neuroprotective roles in SCI. When embedded in polydopamine-based scaffolds, curcumin attenuated inflammation and oxidative stress by inhibiting NF-κB and promoting JAK/STAT signaling, thus enhancing axonal regeneration [187]. It also reduced neuropathic pain, improved motor function, and increased antioxidant markers (SOD, catalase, GABA-A) while lowering lipid peroxidation and tissue damage in SCI rats [188]. A network pharmacology study identified matrix metalloproteinase-9 (MMP9) as a critical curcumin target, with curcumin downregulating its expression and conferring protection in cellular SCI models [189].
5.5 Traumatic Brain Injury
Traumatic brain injury (TBI) remains a critical cause of disability and mortality worldwide, leading to cognitive, emotional, and motor dysfunction [190]. While no definitive pharmacological therapy exists to reverse secondary brain damage, a growing body of evidence supports the role of phytochemicals as neuroprotective agents. These natural compounds, derived from plants, exhibit anti-inflammatory, antioxidant, anti-apoptotic, and neurogenic properties. Here, we explore the classification and mechanisms of action of key phytochemicals studied in the context of TBI.
5.5.1 Polyphenols
Polyphenolic compounds, particularly curcumin and resveratrol, exhibit strong neuroprotective effects in TBI by modulating oxidative stress, inflammation, apoptosis, and autophagy. Advanced formulations and derivatives enhance their bioavailability and therapeutic outcomes.
Curcumin, a major turmeric polyphenol, offers multifaceted neuroprotection in TBI by targeting oxidative stress and neuroinflammation. Nanoparticle formulations like CAQK-modified C-PPS/C enhanced brain targeting, reduced ROS and NF-κB activity, preserved BBB integrity, and improved neurological recovery [191]. Preventive use of turmeric extract in repetitive TBI reduced TNF-α, GFAP, p-Tau, and TDP-43 levels [192]. Derivatives such as bisdemethoxycurcumin (BDMC) and tetrahydrocurcumin (THC) further modulated autophagy and microglial polarization through the HSP90AA1/TFEB/Nrf2 and GSK3β/PTEN/PI3K/Akt pathways, respectively, reducing inflammation and apoptosis.
Resveratrol (RSV) also shows strong efficacy in TBI. Functionalized silver nanowire MXene biopatches combining RSV enabled real-time GFAP monitoring and reduced inflammation [195]. RSV downregulated ER stress proteins (CHOP, GRP78), pro-inflammatory cytokines (TNF-α, IL-1β), and oxidative stress markers (MDA), while upregulating GSH and maintaining hippocampal architecture [196,197].
5.5.2 Terpenoids
Withaferin A (WFA) from Withania somnifera improved neurobehavior, reduced BBB disruption, edema, and endothelial apoptosis, while decreasing IL-1β, IL-6, TNF-α and microglial activation [198]. Ginkgolide A (GA) from Ginkgo biloba enhanced neurological function, decreased oxidative stress (MDA, 8-OHdG), and inhibited apoptosis, supported by increased SOD activity [199]. Ginsenoside Rg3, from ginseng, reduced brain edema, microglial inflammation, and hippocampal damage by activating SIRT1 and inhibiting NF-κB; its effect was blocked by SIRT1 inhibitor NAM, confirming pathway involvement [200].
5.5.3 Alkaloids
Oxyberberine (OBB), a berberine derivative, formulated as nanocrystals (OBB-NC), attenuated neuroinflammation via HMGB1/TLR4/NF-κB pathway inhibition. It improved cognitive, anxiety, and depression-like behaviors post-TBI and suppressed oxidative stress and nitric oxide production [201].
5.5.4 Flavonoids
Quercetin, a common dietary flavonoid, demonstrated broad neuroprotection by targeting oxidative stress, inflammation, ferroptosis, and apoptosis. Network pharmacology identified hub genes (e.g., HIF1A, IL6, TP53), and pathways (HIF-1, PI3K-Akt, IL-17) as key mediators [202]. In vivo, quercetin suppressed microglial activation, reduced edema and neuronal death, and acted via the PGC-1α/Nrf2 pathway while inhibiting HDAC3 nuclear translocation [203].
5.5.5 Phenolic Acids
Ferulic acid (FA), a key component of traditional formulas like Guanxin II and DCH, modulated the gut-brain axis, reduced GI dysfunction, and suppressed neuroinflammation through Ghrelin/GHSR signaling and inhibition of the TLR4/NF-κB/NLRP3 pathway [204]. It also protected the BBB, decreased brain edema, and influenced HPA axis hormones including dopamine, serotonin, and BDNF, further validating its role in holistic neuroprotection [205].
6. Interactions between Phytochemicals and Allopathic Drugs
Natural compounds, including herbal medicines and dietary phyto-compounds, have increasingly been utilized for therapeutic synergy with conventional drugs used to treat various brain disorders. Such combinational approaches capitalize on antioxidant, anti-inflammatory, neuroprotective, and immune-boosting properties inherent to these natural substances. However, these combinations can also lead to unintended pharmacokinetic or pharmacodynamic interactions, significantly influencing therapeutic outcomes.
Flavonoids such as quercetin and silymarin are prominent for modulating transporter proteins like P-glycoprotein (P-gp), affecting the brain availability of several CNS drugs. High doses of quercetin inhibit P-gp, enhancing brain levels of medications like ritonavir and quinidine, while low doses may activate efflux mechanisms, decreasing uptake of agents such as vincristine [206-208]. Procyanidins from pine bark similarly inhibit P-gp at the blood-brain barrier (BBB), facilitating greater accumulation of chemotherapeutic drugs within brain tissues, which is particularly beneficial in managing brain tumors [209].
Curcumin, another polyphenol, notably boosts the delivery and efficacy of brain-targeted therapeutics when administered via nanoparticle systems. For example, curcumin-loaded nanoparticles significantly improve brain localization of doxorubicin, thereby enhancing its effectiveness against brain cancers through P-gp inhibition [210]. Additionally, curcumin demonstrates pharmacodynamic synergy with antiepileptic drugs, improving cognitive and neuroprotective outcomes without altering systemic drug levels [211].
Compounds like borneol, known for their membrane-permeabilizing properties, have been used to enhance CNS drug delivery. When incorporated into nanoparticles or niosomes, borneol significantly increases the brain uptake of drugs like dopamine, ginkgolide B, and puerarin, suggesting an improved therapeutic potential in models of Parkinson’s disease [212,213].
Terpenoids in Ginkgo biloba (GB) extracts also influence drug metabolism. GB has shown dual effects—enhancing midazolam exposure while reducing carbamazepine plasma levels—likely due to the interplay between its terpene lactones and flavonol glycosides on CYP3A4 activity [214,215].
Compounds such as hyperforin from St. John’s Wort (SJW) induce both CYP3A4 and P-gp expression, leading to reduced plasma concentrations of drugs like amitriptyline and docetaxel, and thus diminishing their therapeutic effects [216,217]. Variations in SJW extract composition further impact interaction severity, underlining the importance of standardized formulations.
In contrast, grapefruit juice, rich in furanocoumarins, inhibits CYP3A4, notably increasing plasma levels of carbamazepine, a common antiepileptic. This interaction can enhance therapeutic outcomes but also raises toxicity risks if not monitored carefully [218]. Ginsenosides—particularly protopanaxadiol derivatives from Ginseng—demonstrate both pharmacodynamic and pharmacokinetic interactions. Co-administration with carbamazepine in liver microsome studies showed enhanced CYP3A4-mediated metabolism, potentially reducing therapeutic exposure [219]. These findings suggest caution in the simultaneous use of Ginseng products with drugs primarily metabolized by CYP3A4. Imperatorin, found in Angelica sinensis and Angelica dahurica, enhances diazepam levels by inhibiting its hepatic metabolism, reducing clearance, and increasing systemic exposure [220]. Similar effects are seen with Khat (Catha edulis), a CNS stimulant, which inhibits both CYP3A4 and CYP2D6—enzymes critical for the metabolism of antipsychotics like aripiprazole and antidepressants such as clomipramine [221].
Melatonin exerts notable pharmacodynamic synergy with antiepileptic drugs such as carbamazepine and phenytoin. These effects are independent of melatonin's influence on drug plasma levels, pointing instead to its intrinsic neuroprotective and modulatory properties on neuronal excitability [222,223]. Caffeine, although widely consumed, may interfere with epilepsy management. It has been shown to diminish the anticonvulsant efficacy of several antiepileptics including carbamazepine, valproate, and topiramate, via pharmacodynamic mechanisms rather than alterations in systemic concentrations [224]. Quecertin stands out for its ability to boost the cytotoxic effects of doxorubicin and temozolomide in brain tumor models through inhibition of heat shock protein expression. This pharmacodynamic enhancement suggests promising avenues for adjunctive therapy in neuroblastoma and astrocytoma [225,226].
While the therapeutic synergy between phytochemicals and allopathic drugs holds considerable promise for treating neurological conditions, the associated interactions are complex and multifactorial. These effects depend on numerous factors, including dosage, compound structure, metabolic pathways, and individual variability. Therefore, it is essential to undertake more rigorous clinical evaluations, employ standardized plant extracts, and utilize advanced pharmacokinetic modeling to mitigate risks and maximize benefits. Such strategies will ensure the safe and effective integration of phytochemicals into neurological disease management protocols.
7. Conclusions
The intersection of phytochemicals and allopathic therapy presents a promising new frontier in the treatment of neurological diseases. Conditions such as Alzheimer’s disease, Parkinson’s disease, epilepsy, spinal injuries, and traumatic brain injuries continue to pose significant clinical challenges, often characterized by limited therapeutic efficacy and severe side effects. Although allopathic treatments provide essential symptom relief and disease management, they frequently lack the capacity for neuroprotection or regeneration.
Phytochemicals—natural compounds derived from plants, including flavonoids, alkaloids, and terpenes—have demonstrated antioxidant, anti-inflammatory, and neuroprotective properties in both experimental and clinical studies. These phytochemicals have the potential to augment pharmaceutical interventions, thereby enhancing therapeutic effects or mitigating undesirable side effects. However, the co-administration of phytochemicals may also introduce risks, such as adverse drug-drug interactions, alterations in drug metabolism, or reduced therapeutic efficacy. Therefore, a comprehensive understanding of phytochemical interactions with conventional neurologic medications is imperative.
The integration of plant-based agents into clinical therapy requires rigorous research, standardization, and meticulous consideration of their efficacy and safety. As the body of scientific evidence grows, an expanded holistic and integrative approach to neurologic therapeutics—utilizing the strengths of both phytotherapy and contemporary pharmacy—has the potential to transform therapeutic outcomes for patients and broaden the arsenal available for addressing neurodegenerative and neurotraumatic disorders.
Funding: The research work of DKA is supported by the R25AI179582 grant from the National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Competing interests: All authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication: All authors have read the manuscript and consented for publication.
References
- Rajmohan R, Reddy PH. Amyloid Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J Alzheimers Dis 57 (2017): 975-999.
- Serrano-Pozo A, Das S, Hyman BT. APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol 20 (2021): 68-80.
- Wang C, Zong S, Cui X, et al. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front Immunol 14 (2023): 1117172.
- Ramesh S, Arachchige ASPM. Depletion of dopamine in Parkinson’s disease and relevant therapeutic options: A review of the literature. AIMS Neurosci 10 (2023): 200-231.
- Gómez-Benito M, Granado N, García-Sanz P, et al. Moratalla, Modeling Parkinson’s Disease with the Alpha-Synuclein Protein. Front. Pharmacol 11 (2020).
- Aarsland D, Batzu L, Halliday GM, et al. Weintraub, Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 7 (2021) 47.
- Radad K, Moldzio R, Krewenka C, et al. Pathophysiology of non-motor signs in Parkinsons disease: some recent updating with brief presentation. Explor Neuroprot Ther 3 (2023): 24-46.
- Nielsen SD, Pearson NM, Seidler K. The link between the gut microbiota and Parkinson’s Disease: A systematic mechanism review with focus on α-synuclein transport. Brain Research 1769 (2021): 147609.
- Stafstrom CE, Carmant L. Seizures and Epilepsy: An Overview for Neuroscientists. Cold Spring Harb Perspect Med 5 (2015): a022426.
- Blumenfeld H. Epilepsy and the Consciousness System: Transient Vegetative State?, Neurol Clin 29 (2011): 801-823.
- Veerabathiran R, Iyshwarya BK. Identification of novel epilepsy genetics and development of advanced diagnostic approaches, Brain Disorders 17 (2025): 100202.
- Mendoza-Mari Y, Rai V, Radwan MM, et al. Modulation of Inflammatory Response by Electromagnetic Field Stimulation in Traumatic Brain Injury in Yucatan Swine, J Surg Res (Houst) 7 (2024): 20-40.
- Freire MAM, Rocha GS, Bittencourt LO, et al. Cavalcanti, Cellular and Molecular Pathophysiology of Traumatic Brain Injury: What Have We Learned So Far?, Biology (Basel) 12 (2023): 1139.
- Rai V, Mendoza-Mari Y, Brazdzionis J, et al. Transcriptomic Analysis of Gene Expression and Effect of Electromagnetic Field in Brain Tissue after Traumatic Brain Injury, Journal of Biotechnology and Biomedicine 7 (2024): 101-110.
- Kiraly MA, Kiraly SJ. Traumatic Brain Injury and Delayed Sequelae: A Review - Traumatic Brain Injury and Mild Traumatic Brain Injury (Concussion) are Precursors to Later-Onset Brain Disorders, Including Early-Onset Dementia, The Scientific World Journal 7 (2007): 193268.
- Blennow K, Brody DL, Kochanek PM, et al. Zetterberg, Traumatic brain injuries, Nat Rev Dis Primers 2 (2016) 16084.
- Wilson L, Stewart W, Dams-O’Connor K, et al. Polinder, The chronic and evolving neurological consequences of traumatic brain injury, The Lancet Neurology 16 (2017): 813-825.
- Hossain MDS, Wazed MA, Asha S, et al. Dietary Phytochemicals in Health and Disease: Mechanisms, Clinical Evidence, and Applications—A Comprehensive Review, Food Science & Nutrition 13 (2025): e70101.
- Maria do Socorro S, Behrens MD, Moragas-Tellis CJ, Get al. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds, Oxid Med Cell Longev 2022 (2022): 9966750.
- Kumar A, Kumar NPM, Jose A, et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method, Molecules 28 (2023) 887.
- Thakur M, Singh K, Khedkar R. 11 - Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues, In: Prakash B (Ed.), Functional and Preservative Properties of Phytochemicals, Academic Press (2020): 341-361.
- Doughari JH. Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents, in: Phytochemicals - A Global Perspective of Their Role in Nutrition and Health. IntechOpen (2012).
- Connolly EL, Sim M, Travica N, et al. Glucosinolates From Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front Pharmacol 12 (2021): 767975.
- Saeedi M, Mehranfar F. Challenges and Approaches of Drugs Such as Memantine, Donepezil, Rivastigmine, and Aducanumab in the Treatment, Control and Management of Alzheimer’s Disease. Recent Patents on Biotechnology 16 (2022): 102-121.
- Khanam FM. Donepezil to lecanemab-advancements in targeted therapy of Alzheimer’s disease so far, International Journal of Advances in Medicine 10 (2023): 653-659.
- Thangwaritorn S, Lee C, Metchikoff E, et al. A Review of Recent Advances in the Management of Alzheimer’s Disease, Cureus 16 (2024): e58416.
- Marsool MDM, Prajjwal P, Reddy YB, et al. Newer modalities in the management of Alzheimer’s dementia along with the role of aducanumab and lecanemab in the treatment of its refractory cases. Disease-a-Month 69 (2023): 101547.
- Buccellato FR, D’Anca M, Tartaglia GM, et al. Treatment of Alzheimer’s Disease: Beyond Symptomatic Therapies, International Journal of Molecular Sciences 24 (2023): 13900.
- Ameen TB, Kashif SN, Abbas SMI, et al. Unraveling Alzheimer’s: the promise of aducanumab, lecanemab, and donanemab, Egypt J Neurol Psychiatry Neurosurg 60 (2024): 72.
- Rahman A, Banu Z, Rani R, et al. Unravelling Alzheimer’s disease: Insights into pathophysiology, etiology, diagnostic approaches, and the promise of aducanumab, lecanemab, and donanemab, J. Phytonanotech Pharmaceut Sci 4 (2024).
- Thawabteh AM, Ghanem AW, AbuMadi S, et al. Recent Advances in Therapeutics for the Treatment of Alzheimer’s Disease, Molecules 29 (2024): 5131.
- Gorthi SP, Gupta D. Alzheimer’s Disease: Treatment Today and Tomorrow, Annals of Indian Academy of Neurology 26 (2023): 326.
- Ferraiolo M, Hermans E. The complex molecular pharmacology of the dopamine D2 receptor: Implications for pramipexole, ropinirole, and rotigotine. Pharmacology & Therapeutics 245 (2023): 108392.
- Pringsheim T, Day GS, Smith DB, et al. Dopaminergic Therapy for Motor Symptoms in Early Parkinson Disease Practice Guideline Summary. Neurology 97 (2021): 942-957.
- Alonso Cánovas A, Luquin Piudo R, García Ruiz-Espiga P, et al. Dopaminergic agonists in Parkinson’s disease. Neurología (English Edition) 29 (2014): 230-241.
- Ferraiolo M, Hermans E. The complex molecular pharmacology of the dopamine D2 receptor: Implications for pramipexole, ropinirole, and rotigotine. Pharmacology & Therapeutics 245 (2023): 108392.
- Hakami T. Efficacy and tolerability of antiseizure drugs. Ther Adv Neurol Disord 14 (2021): 17562864211037430.
- Hakami T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacology Reports 41 (2021): 336-351.
- Sáenz-Farret M, Tijssen MAJ, Eliashiv D, et al. Antiseizure Drugs and Movement Disorders, CNS Drugs 36 (2022): 859-876.
- Greenfield LJ. Molecular Mechanisms of Antiseizure Drug Activity at GABAA Receptors. Seizure 22 (2013): 589-600.
- Sánchez JD, Gómez-Carpintero J, González JF, et al. Twenty-first century antiepileptic drugs. An overview of their targets and synthetic approaches, European Journal of Medicinal Chemistry 272 (2024): 116476.
- Rossi R, Arjmand S, Bærentzen SL, et al. Synaptic Vesicle Glycoprotein 2A: Features and Functions. Front Neurosci 16 (2022).
- Contreras-García IJ, Cárdenas-Rodríguez N, Romo-Mancillas A, et al. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals (Basel) 15 (2022): 475.
- Czapinska-Ciepiela EK, Luszczki J, Czapinski P, et al. Presynaptic antiseizure medications - basic mechanisms and clues for their rational combinations, Pharmacol. Rep 76 (2024): 623-643.
- Liu LJW, Rosner J, Cragg JJ. Journal Club: High-dose methylprednisolone for acute traumatic spinal cord injury. Neurology 95 (2020): 272-274.
- Dimitrov N, Kemle K. Palliative and Serious Illness Patient Management for Physician Assistants. Oxford University Press (2021).
- Rabchevsky AG, Kitzman PH. Latest Approaches for the Treatment of Spasticity and Autonomic Dysreflexia in Chronic Spinal Cord Injury. Neurotherapeutics 8 (2011): 274-282.
- Marcianò G, Vocca C, Evangelista M, et al. The Pharmacological Treatment of Chronic Pain: From Guidelines to Daily Clinical Practice. Pharmaceutics 15 (2023): 1165.
- Patel T. Pharmacologic Treatments for CRPS, in: E.F. Lawson, J.P. Castellanos (Eds.), Complex Regional Pain Syndrome: A Clinical Guide. Springer International Publishing Cham (2021): 65-78.
- Mackey S, Feinberg S. Pharmacologic Therapies for Complex Regional Pain Syndrome, Curr Pain Headache Rep 11 (2007): 38-43.
- Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns & Trauma 5 (2017).
- Brazdzionis J, Radwan MM, Thankam F, et al. A Swine Model of Traumatic Brain Injury: Effects of Neuronally Generated Electromagnetic Fields and Electromagnetic Field Stimulation on Traumatic Brain Injury-Related Changes. Cureus 15 (2023): e42544.
- Tani J, Wen YT, Hu CJ, et al. Current and Potential Pharmacologic Therapies for Traumatic Brain Injury. Pharmaceuticals 15 (2022): 838.
- Candy N, Tsimiklis C, Poonnoose S, et al. The use of antiepileptic medication in early post traumatic seizure prophylaxis at a single institution, J Clin Neurosci 69 (2019): 198-205.
- Dekundy A, Pichler G, El Badry R, et al. Amantadine for Traumatic Brain Injury—Supporting Evidence and Mode of Action, Biomedicines 12 (2024): 1558.
- Johansson B, Andréll P, Rönnbäck Lars, et al. Follow-up after 5.5 years of treatment with methylphenidate for mental fatigue and cognitive function after a mild traumatic brain injury. Brain Injury 34 (2020): 229-235.
- Blum B, Kaushal S, Khan S, et al. Melatonin in Traumatic Brain Injury and Cognition, Cureus (2021).
- Thomas M, Banks C, Ray A. The MRC CRASH trial at 20: Time to reappraise corticosteroids for traumatic brain injury?, J Intensive Care Soc 26 (2025): 277.
- Czekajlo MS, Milbrandt EB. Corticosteroids increased short and long-term mortality in adults with traumatic head injury, Crit Care 9 (2005): E21.
- Hicks AJ, Clay FJ, James AC, et al. Effectiveness of Pharmacotherapy for Depression after Adult Traumatic Brain Injury: an Umbrella Review, Neuropsychol Rev 33 (2023): 393-431.
- Yue JK, Burke JF, Upadhyayula PS, et al. Selective Serotonin Reuptake Inhibitors for Treating Neurocognitive and Neuropsychiatric Disorders Following Traumatic Brain Injury: An Evaluation of Current Evidence. Brain Sci 7 (2017): 93.
- Susanto M, Pangihutan Siahaan AM, Wirjomartani BA, et al. The neuroprotective effect of statin in traumatic brain injury: A systematic review. World Neurosurg X 19 (2023): 100211.
- Stein DG. Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience 191 (2011): 101-106.
- Aychman MM, Goldman DL, Kaplan JS. Cannabidiol’s neuroprotective properties and potential treatment of traumatic brain injuries. Front Neurol 14 (2023): 1087011.
- Liu X, Baxley S, Hebron M, et al. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease, Int J Mol Sci 26 (2025): 5044.
- Gu J, Li Z, Chen H, et al. Neuroprotective Effect of Trans-Resveratrol in Mild to Moderate Alzheimer Disease: A Randomized, Double-Blind Trial, Neurol Ther 10 (2021): 905-917.
- Zarneshan SN, Fakhri S, Kiani A, et al. Polydatin attenuates Alzheimer’s disease induced by aluminum chloride in rats: evidence for its antioxidant and anti-inflammatory effects. Front Pharmacol 16 (2025) 1574323.
- Malaiya A, Kenwat R, Mamgain A, et al. Intranasal resveratrol delivery to the brain with chitosan-decorated bovine serum albumin nanoparticles: Advancing Alzheimer’s management in old female rats through QbD-based optimization, in vitro evaluation, and in vivo exploration. International Journal of Biological Macromolecules 311 (2025): 143300.
- Tao G, Wang X, Wang J, et al. Dihydro-resveratrol ameliorates NLRP3 inflammasome-mediated neuroinflammation via Bnip3-dependent mitophagy in Alzheimer’s disease, Br J Pharmacol 182 (2025): 1005-1024.
- Yang H, Zeng F, Luo Y, et al. Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research, Molecules 27 (2022): 3879.
- Pawar S, Suvarna V. Curcumin-loaded PEG-functionalized carbon nanotubes: a novel strategy for Alzheimer’s management, Ther Deliv (2025): 1-9.
- Malek SZ, Arasteh A. In Vitro Regulation of Amyloid production in Alzheimer’s Disease via Curcumin-Loaded Silver Nanoparticles with Antioxidant and Acetylcholinesterase-Inhibiting Properties. Mol Neurobiol (2025).
- Li J, Huang D, Liao W, et al. Advanced Nanopharmaceutical Intervention for the Reduction of Inflammatory Responses and the Enhancement of Behavioral Outcomes in APP/PS1 Transgenic Mouse Models. Pharmaceutics 17 (2025): 177.
- Ding YN, Li MQ, Song JY, et al. Engineered exosomes improve the effects of curcumin on protecting mitochondria of neurons in Alzheimer’s disease, Mater Today Bio 32 (2025): 101738.
- Yang D, Deng Z, Zhou H, et al. Exosome-mediated dual drug delivery of curcumin and methylene blue for enhanced cognitive function and mechanistic elucidation in Alzheimer’s disease therapy. Front Cell Dev Biol 13 (2025): 1562565.
- Bashirrohelleh MA, Bavarsad K, Khodadadi A, M. et al. Curcumin-enhanced stem cell exosomes: A novel approach to modulating neuroinflammation and improving cognitive function in a rat model of Alzheimer’s disease, Eur J Pharmacol 999 (2025): 177695.
- Awad SM, Attia YA, ElSayed H, et al. Efficacy of curcumin-selenium nanoemulsion in alleviating oxidative damage induced by aluminum chloride in a rat model of Alzheimer’s disease. J Mol Histol 56 (2025): 122.
- Yang H, Li K, Wang T, et al. Novel Dual-Functional Half-Curcumin Analogues as a Fluorescent and PET Probe for β-Amyloid Imaging in the Alzheimer’s Disease APP/PS1 Model, ACS Chem Neurosci 16 (2025): 365-373.
- Strumentova ES, Lobzin VY, Maltsev DS, et al. Curcumin-induced increased retinal fluorescence as a new method in the early diagnosis of Alzheimer’s disease. Zh Nevrol Psikhiatr Im S S Korsakova 125 (2025): 19-25.
- Rodrigues B, Ventura E, Moreira P, et al. New low-dose curcumin derivative with therapeutic potential in Alzheimer’s disease: Results from an in vitro and in vivo study in mice. Neurobiol Aging 147 (2025): 105-123.
- Satpathy B, Rajwar TK, Mishra A, et al. Intra-nasal Delivery of Quercetin-Loaded Nanostructured Lipid Carriers (NLCs) in the Management of Alzheimer’s Disease: Formulation, In Vitro and In Vivo assessment, Mol Neurobiol (2025).
- Awad AS, El-Mokadem BM, Sherif MM, et al. Can Quercetin protect against the pre-disposing factors for Alzheimer’s disease via inhibiting NLRP3 inflammasome pathway?. J Pharm Pharmacol (2025): rgaf020.
- Al-Sawasany AS, Fayed HM, Mahmoud BF, et al. Evaluation of the Neurotherapeutic Effect of Quercetin on Neuronal miR-124 and β-Site Amyloid Precursor Protein Cleaving Enzyme 1 (BACE1) in an Experimental Alzheimer’s Disease Model, J Biochem Mol Toxicol 39 (2025): e70290.
- Bianchini MC, Franscescon F, Soares A, et al. Potential Benefits of Quercetin through P2X7 Modulation against Neuroinflammation in Alzheimer’s Disease, Curr Neuropharmacol (2025).
- Fang C, Chen Q, Zheng G, et al. Cellulose-like chitosan microfibrils facilitate targeted release and enhance the prolonged residence time of quercetin-selenium nanoparticles for Alzheimer’s disease treatment. Int J Pharm 670 (2025): 125-129.
- Chetia P, Mazumder MK, Mahanta S, et al. A novel phytochemical from Dipteris wallichii inhibits human β-secretase 1: Implications for the treatment of Alzheimer’s disease, Medical Hypotheses 143 (2020): 109839.
- Sun C, Dong S, Chen W, et al. Berberine alleviates Alzheimer’s disease by regulating the gut microenvironment, restoring the gut barrier and brain-gut axis balance. Phytomedicine 129 (2024): 155624.
- Wang C, Zou Q, Pu Y, et al. Berberine Rescues D-Ribose-Induced Alzheimer‘s Pathology via Promoting Mitophagy, International Journal of Molecular Sciences 24 (2023): 5896.
- Liu JY, Dai Y, He YX, et al. Effect of berberine on cognitive function and β-amyloid precursor protein in Alzheimer’s disease models: a systematic review and meta-analysis. Front Pharmacol 14 (2024).
- Shalaby AM, Alnasser SM, Ahmed Khairy D, et al. The neuroprotective effect of ginsenoside Rb1 on the cerebral cortex changes induced by aluminium chloride in a mouse model of Alzheimer’s disease: A histological, immunohistochemical, and biochemical study. Journal of Chemical Neuroanatomy 129 (2023): 102248.
- Wang Y, Li Y, Yang W, et al. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer’s disease induced by Aβ1-40, Am J Transl Res 10 (2018): 796-805.
- Liu GZ, Niu TT, Yu Q, et al. Ginkgolide attenuates memory impairment and neuroinflammation by suppressing the NLRP3/caspase-1 pathway in Alzheimer’s disease. Aging (Albany NY) 15 (2023): 10237-10252.
- Dindar Z, Anbaraki A, Hosseini SS, et al. The Use of Natural Volatile Compounds on the Fibrillation Domain of Amyloid Beta (GSNKGAIIGLM)-Towards Promising Agents to Combat Alzheimer’s Disease. ACS Chem Neurosci 16 (2025): 1086-1102.
- Anbaraki A, Dindar Z, Mousavi-Jarrahi Z, et al. The novel anti-fibrillary effects of volatile compounds α-asarone and β-caryophyllene on tau protein: Towards promising therapeutic agents for Alzheimer’s disease, International Journal of Biological Macromolecules 271 (2024): 132401.
- Jia M, Ning F, Wen J, et al. Secoisolariciresinol diglucoside attenuates neuroinflammation and cognitive impairment in female Alzheimer’s disease mice via modulating gut microbiota metabolism and GPER/CREB/BDNF pathway, Journal of Neuroinflammation 21 (2024): 201.
- Sasikumar DSN, Thiruselvam P, Sundararajan V, et al. Insights into dietary phytochemicals targeting Parkinson’s disease key genes and pathways: A network pharmacology approach. Computers in Biology and Medicine 172 (2024): 108195.
- Chen M, Peng L, Gong P, et al. Baicalein Induces Mitochondrial Autophagy to Prevent Parkinson’s Disease in Rats via miR-30b and the SIRT1/AMPK/mTOR Pathway. Front Neurol 12 (2022): 646817.
- Huang J, Zhang X, Yang X, et al. Baicalin exerts neuroprotective actions by regulating the Nrf2-NLRP3 axis in toxin-induced models of Parkinson’s disease, Chemico-Biological Interactions 387 (2024): 110820.
- Balakrishnan R, Azam S, Cho DY, et al. Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson’s Disease: Current Knowledge and Future Perspectives, Oxidative Medicine and Cellular Longevity 2021 (2021): 680935.
- Özduran G, Becer E, Vatansever HS, et al. Neuroprotective effects of catechins in an experimental Parkinson’s disease model and SK-N-AS cells: evaluation of cell viability, anti-inflammatory and anti-apoptotic effects. Neurol Res 44 (2022): 511-523.
- Cheng CY, Barro L, Tsai ST, et al. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection, Int J Mol Sci 22 (2021): 3037.
- Tseng HC, Wang MH, Chang KC, et al. Protective Effect of (-) Epigallocatechin-3-gallate on Rotenone-Induced Parkinsonism-like Symptoms in Rats. Neurotox Res 37 (2020): 669-682.
- Yousefi M, Mirshekar MA, Afsharfar M, et al. Neuroprotective effects of quercetin on motor impairments and anxiety-like behaviors in a rat model of Parkinson’s disease, Behav Brain Res 493 (2025): 115692.
- Azzolin VF, Azzolin VF, Ribeiro EE, et al. Synergistic Neuroprotective and Immunomodulatory Effects of Cocoa Seed Husk and Guarana Extract: A Nutraceutical Approach for Parkinson’s Disease Management. Antioxidants (Basel) 14 (2025): 348.
- Li Y, Man M, Tian Y, et al. Chang, Quercetin protects against neuronal toxicity by activating the PI3K/Akt/GSK-3β pathway in vivo models of MPTP-induced Parkinson’s disease. Inflammopharmacology (2025).
- Zhang L, Zhan M, Sun H, et al. Mesenchymal Stem-Cell-Derived Exosomes Loaded with Phosphorus Dendrimers and Quercetin Treat Parkinson’s Disease by Modulating Inflammatory Immune Microenvironment, ACS Appl Mater Interfaces 17 (2025): 32013-32027.
- Revankar AA, Patil AS, Karishetti R, et al. Enhanced bioavailability of Quercetin-loaded niosomal in situ gel for the management of Parkinson’s disease. Front Pharmacol 15 (2024): 1519649.
- Owona BA, Ngoungoure VN, Tchamba JT, et al. Cola acuminata extract’s inhibition of NLRP3 inflammasome in THP-1 cells as a potential treatment option for Parkinson’s disease. Metab Brain Dis 40 (2025): 229.
- Huang YB, Tian LL, Zhu ZW, et al. Apigenin enhances Nrf2-induced chaperone-mediated autophagy and mitigates α-synuclein pathology: Implications for Parkinson’s disease therapy. Phytomedicine 141 (2025): 156652.
- Liu K, An J, Zhang J, et al. Network pharmacology combined with experimental validation show that apigenin as the active ingredient of Campsis grandiflora flower against Parkinson’s disease by inhibiting the PI3K/AKT/NF-κB pathway, PLoS One 19 (2024): e0311824.
- Yarim GF, Kazak F, Yarim M, et al. Apigenin alleviates neuroinflammation in a mouse model of Parkinson’s disease, Int J Neurosci 135 (2025): 505-514.
- Shukla R, Mishra K, Singh S. Exploring therapeutic potential of Bacopa monnieri bioactive compounds against Alzheimer’s and Parkinson’s diseases. 3 Biotech 15 (2025): 61.
- de Jesus LB, Frota AF, de Araújo FM, et al. Effect of the Flavonoid Rutin on the Modulation of the Myenteric Plexuses in an Experimental Model of Parkinson’s Disease. Int J Mol Sci 25 (2024): 1037.
- Khan A, Ullah F, Alkreathy HM, et al. Phytochemical screening, antioxidant and anti-Parkinson activities of Berula erecta: A novel medicinal plant, PLoS One 19 (2024): e0305751.
- Abolaji AO, Adedara AO, Madu JC, et al. Aschner, Experimental and computational insights into the therapeutic mechanisms of resveratrol in a Drosophila α-synuclein model of Parkinson’s disease. Sci Rep 15 (2025): 17769.
- Chiang MC, Yang YP, Nicol CJB, et al. Resveratrol-Enhanced Human Neural Stem Cell-Derived Exosomes Mitigate MPP+-Induced Neurotoxicity Through Activation of AMPK and Nrf2 Pathways and Inhibition of the NLRP3 Inflammasome in SH-SY5Y Cells, Life (Basel) 15 (2025): 294.
- Yang JT, Kuo YC, Lee KC, et al. Resveratrol and ceftriaxone encapsulated in hybrid nanoparticles to prevent dopaminergic neurons from degeneration for Parkinson’s disease treatment. Biomater Adv 166 (2025): 214065.
- Bandiwadekar A, Jose J, Gopan G, et al. Transdermal delivery of resveratrol loaded solid lipid nanoparticle as a microneedle patch: a novel approach for the treatment of Parkinson’s disease. Drug Deliv Transl Res 15 (2025): 1043-1073.
- Feng S, Gui J, Qin B, et al. Resveratrol Inhibits VDAC1-Mediated Mitochondrial Dysfunction to Mitigate Pathological Progression in Parkinson’s Disease Model, Mol Neurobiol 62 (2025): 6636-6654.
- Pislyagin EA, Tarbeeva DV, Yurchenko EA, et a. Neuroprotective Activity of Oligomeric Stilbenes from Alpha Grape Stems in In Vitro Models of Parkinson’s Disease. Int J Mol Sci 26 (2025): 2411.
- Banerjee C, Barman R, Darshani P, et al. α-Viniferin, a dietary phytochemical, inhibits Monoamine oxidase and alleviates Parkinson’s disease associated behavioral deficits in a mice model. Neurochem Int 174 (2024): 105698.
- Teksen Y, Gündüz MK, Berikten D, et al. Peganum harmala L. seed extract attenuates anxiety and depression in rats by reducing neuroinflammation and restoring the BDNF/TrkB signaling pathway and monoamines after exposure to chronic unpredictable mild stress. Metab Brain Dis 39 (2024): 1523-1541.
- Kim JE, Min KS, Go J, et al. Water extract of Humulus japonicus improves age-related cognitive decline by inhibiting acetylcholinesterase activity and the acetylcholine signaling pathway. Mol Med Rep 31 (2025): 131.
- Zajdel P, Matloka M, Konieczny J, et al. Simultaneous 5-HT1BR agonist/5-HT6R antagonist action as a potential treatment of Parkinson’s disease and its comorbidities. J Pharmacol Exp Ther 392 (2025): 100055.
- He MT, Shin YS, Kim HY, et al. Carthamus tinctorius seeds-Taraxacum coreanum combination attenuates scopolamine-induced memory deficit through regulation of inflammatory response and cholinergic function, Nutr Res Pract 18 (2024): 647-662.
- Tinku, Choudhary S. Hydroxycinnamic acids mediated modulation of α-Synuclein fibrillation: Biophysical insights. Biochem Biophys Res Commun 744 (2025): 151195.
- Hedayatikatouli F, Kalyn M, Elsaid D, et al. Neuroprotective Effects of Ascorbic Acid, Vanillic Acid, and Ferulic Acid in Dopaminergic Neurons of Zebrafish. Biomedicines 12 (2024): 2497.
- Youssef OM, Lashine NH, El-Nablaway M, et al. Ferulic acid mitigated rotenone toxicity -Evoked Parkinson in rat model by featuring apoptosis, oxidative stress, and neuroinflammation signaling. Tissue Cell 91 (2024): 102614.
- Li Y, Shi R, Xia L, et al. Identification of Key Active Constituents in Eucommia ulmoides Oliv. Leaves Against Parkinson’s Disease and the Alleviative Effects via 4E-BP1 Up-Regulation. Int J Mol Sci 26 (2025): 2762.
- Xu J, Dai P, Zhang C, et al. Injectable Hierarchical Bioactive Hydrogels with Fibroblast Growth Factor 21/Edaravone/Caffeic Acid Asynchronous Delivery for Treating Parkinson’s Disease. Adv Sci (Weinh) 12 (2025): e2412020.
- Kumar J, Delgado SA, Sarma H, et al. Caffeic acid recarbonization: A green chemistry, sustainable carbon nano material platform to intervene in neurodegeneration induced by emerging contaminants. Environ Res 237 (2023): 116932.
- Papagiouvannis G, Theodosis-Nobelos P, A. Rekka. Trolox, Ferulic, Sinapic, and Cinnamic Acid Derivatives of Proline and GABA with Antioxidant and/or Anti-Inflammatory Properties. Molecules 29 (2024): 3763.
- van Eeghen AM, Thiele EA, Amin S, et al. Protocol for EpiCom: A phase 3b/4 study of behavioral outcomes following adjunctive cannabidiol for the management of tuberous sclerosis complex-associated neuropsychiatric disorders (TAND). PLoS One 20 (2025): e0324648.
- Jackson K, Shabat-Simon M, Bar-On J, et al. The Anticonvulsant Effects of Different Cannabis Extracts in a Zebrafish Model of Epilepsy, Biomolecules 15 (2025): 654.
- de Pieri Pickler K, de Farias ACS, Lodetti G, et al. Cannabidiol Pretreatment Reduces Status Epilepticus and Glutamate Uptake Induced by Kainic Acid in Adult Zebrafish. Cannabis Cannabinoid Res (2025).
- Aizara E, Robin TV, Hanna L, et al. Adjunctive use of cannabidiol in pediatric drug-resistant epilepsy: A retrospective multicenter analysis, Epilepsy Behav 169 (2025): 110426.
- Martimbianco ALC, Silva RB, de O. Cruz Latorraca C, et al. Cannabis derivatives and their synthetic analogs for treatment-resistant epilepsy: A systematic review and meta-analysis. Epilepsy Res 214 (2025) 107559.
- Di Giuseppe LA, Appendino JI, García MDC, et al. Real-life study with pharmaceutical cannabidiol in refractory epilepsy. Rev Fac Cien Med Univ Nac Cordoba 82 (2025): 110-126.
- Ye H, Wan Y, Wang X, et al. Cannabidiol Protects Against Neurotoxic Reactive Astrocytes-Induced Neuronal Death in Mouse Model of Epilepsy, J Neurochem 169 (2025): e70038.
- Johannessen Landmark C, Sætre J, Gottås A, et al. Pharmacokinetic variability and use of therapeutic drug monitoring of cannabidiol in patients with refractory epilepsy, Epilepsia 66 (2025): 1477-1491.
- Hakami AY, Alshehri FS. Therapeutic potential of cannabinoids in neurological conditions: a systematic review of clinical trials. Front Pharmacol 16 (2025): 1521792.
- Yip KL, Udoh M, Sharman LA, et al. Cannabinoid-like compounds found in non-cannabis plants exhibit antiseizure activity in genetic mouse models of drug-resistant epilepsy. Epilepsia 66 (2025): 303-314.
- Xu H, Wu Z, Wang P, et al. (+)-Borneol Enhances the Antiseizure Effects of Retigabine by both Pharmacokinetic and Pharmacodynamic Interaction. Neurochem Res 50 (2025): 147.
- Younis NS, Almostafa MM, Mohamed ME. Geraniol Ameliorates Pentylenetetrazol-Induced Epilepsy, Neuroinflammation, and Oxidative Stress via Modulating the GABAergic Tract: In vitro and in vivo studies. Drug Des Devel Ther 18 (2024): 5655-5672.
- Hashemi P, Mardani P, Eghbali Raz Z, et al. Alpha-Pinene Decreases the Elevated Levels of Astrogliosis, Pyroptosis, and Autophagy Markers in the Hippocampus Triggered by Kainate in a Rat Model of Temporal Lobe Epilepsy. Mol Neurobiol 62 (2025): 2264-2276.
- Li Q, Li R, Ge B, et al. Anticonvulsant effect of Stachydrine on pentylenetetrazole-induced kindling seizure mouse model via Notch and NMDAR signaling pathways. J Ethnopharmacol 349 (2025): 119975.
- Dai WT, Zhu Y, Jiang ZM, et al. Berberine Alleviates Kainic Acid-Induced Acute Epileptic Seizures in Mice via Reshaping Gut Microbiota-Associated Lipid Metabolism. CNS Neurosci Ther 31 (2025): e70253.
- Pradhan P, Rai VK, Halder J, et al. Development and Characterization of Chitosan Nanoparticles Containing Quercetin-β-Cyclodextrin Inclusion Complex for Improved Solubility, Brain Targeting, and Neuroprotective Potential Against Epilepsy. AAPS PharmSciTech 26 (2025): 124.
- Singh Bora K, Kumari K. Evaluation of Anti-epileptic Activity of Cyanthillium cinereum (L.) H. Rob. Leaves in the Experimental Pentylenetetrazole-induced Epileptic Model. Cent Nerv Syst Agents Med Chem (2025).
- Luo C, Wang Z, Liao J, et al. Luteolin ameliorates kainic acid-induced seizure by modulating GADD45B and reducing oxidative stress in hippocampal neurons, Neuropeptides 111 (2025): 102524.
- Niharika DG, Salaria P, Amarendar Reddy. Unraveling potent Glycyrrhiza glabra flavonoids as AKT1 inhibitors using network pharmacology and machine learning-assisted QSAR. Mol Divers (2025).
- Hatem S, Sayyed ME, El-Kayal M. Intranasal delivery of kaempferol via magnesomes for brain seizure treatment: Design, characterization, and biodistribution studies. J Pharm Sci 114 (2025): 103780.
- Socala K, Kowalczuk-Vasilev E, Komar M, et al. Effect of apigenin on seizure susceptibility, parvalbumin immunoreactivity, and GABAA receptor expression in the hippocampal neurons in mice. Eur J Pharmacol 996 (2025): 177548.
- Khan P, Saha N, Nidhi. Neuroprotective effect of naringin by modulation of klotho and HMGB1- TLR4 axis in PTZ-induced kindling in mice. Biochem Biophys Res Commun 742 (2025): 151080.
- Li G, Huang L, Gu D, Pet al. Activity-based chemical proteomics reveals caffeic acid ameliorates pentylenetetrazol-induced seizures by covalently targeting aconitate decarboxylase 1. Cell Commun Signal 23 (2025) 62.
- Amaghnouje A, Chebaibi M, Aldossari SM, et al. Origanum majorana L. polyphenols: in vivo antiepileptic effect, in silico evaluation of their bioavailability, and interaction with the NMDA receptor. Front Chem 11 (2023) 1257769.
- Ciubotaru AD, Leferman CE, Ignat BE, et al. Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish-Pentylenetetrazole (PTZ) Epilepsy Model, Pharmaceuticals (Basel) 18 (2025): 382.
- Hu Y, Qi H, Yang J, et al. Wogonin mitigates microglia-mediated synaptic over-pruning and cognitive impairment following epilepsy, Phytomedicine 135 (2024): 156222.
- Ngaibi J, Bigued, Kandeda AK, et al. Antiepileptic and anti-inflammatory effects of Lippia multiflora moldenke (Verbenaceae) in mice model of chronic temporal lobe epilepsy induced by pilocarpine. Heliyon 10 (2024): e39483.
- Ren R, Zhang M, Shu T, et al. Steroidal Saponins from Water Eggplant (Fruits of Solanum torvum) Exhibit Anti-Epileptic Activity against Pentylenetetrazole-Induced Seizure Model in Zebrafish. Molecules 29 (2024): 1316.
- Zhang JJ, Guan W, Wang Y, et al. Tandem mass tag-based proteomics reveals the antiepileptic mechanism of steroidal saponins from Anemarrhena asphodeloides in Kainic acid induced epileptic rat model. Biomed Chromatogr 38 (2024): e5989.
- Nguyen YND, Jeong JH, Sharma N, et al. Ginsenoside Re protects against kainate-induced neurotoxicity in mice by attenuating mitochondrial dysfunction through activation of the signal transducers and activators of transcription 3 signaling. Free Radic Res 58 (2024): 276-292.
- Li X, Li J, Ji J, et al. Gut microbiota modification by diosgenin mediates antiepileptic effects in a mouse model of epilepsy, J Neurochem 168 (2024): 3982-4000.
- Liu X, Zhao Y, Liang X, et al. In Vivo Evaluation of Self-assembled nano-Saikosaponin-a for Epilepsy Treatment, Mol Biotechnol 66 (2024): 2230-2240.
- Badr NS, El-Nabi SH, El-Gerbed MSA. Impact of Corn Silk Extract on paclitaxel-induced spinal cord and sciatic nerve injuries in rats: Biochemical and histological evaluation. Neurosci Lett 862 (2025): 138282.
- Jing B, Zhao JJ, Chen ZN, et al. ( +)-Catechin Alleviates CCI-Induced Neuropathic Pain by Modulating Microglia M1 and M2 Polarization via the TLR4/MyD88/NF-κB Signaling Pathway. J Neuroimmune Pharmacol 20 (2025): 33.
- Tang W, Wang A, Liu S, et al. Calycosin regulates astrocyte reactivity and astrogliosis after spinal cord injury by targeting STAT3 phosphorylation. J Neuroimmunol 400 (2025): 578535.
- Xiong M, Wang M, Liu X, et al. Quercetin inhibits oligodendrocytes ferroptosis by blocking NCOA4-mediated ferritinophagy, Int Immunopharmacol 150 (2025): 114152.
- Jabbari S, Zakaria ZA, Mohammadi S. Antinociceptive and antineuropathic effects of Trifolium resupinatum L. on formalin-induced nociception and cervical spinal cord hemi-contusion: Underlying Mechanisms. J Ethnopharmacol 337 (2025): 118913.
- Lu Y, Zhou R, Zhu R, et al. Baicalin ameliorates neuroinflammation by targeting TLR4/MD2 complex on microglia via PI3K/AKT/NF-κB signaling pathway. Neuropharmacology 267 (2025): 110296.
- Hai JJ, Liang W, Sun D, et al. Rutin Attenuates Distraction Spinal Cord Injury by Inhibiting Microglial Inflammation Through Downregulation of P38 MAPK/NF-κB/STAT3 Pathway. Mol Neurobiol 62 (2025): 6027-6040.
- Chiu PL, Lin MC, Hsu ST, et al. Rosmarinic acid Ameliorates neuronal regeneration in the bridging silicone rubber conduits of the sciatic nerve in taxol-treated rats. Journal of Traditional and Complementary Medicine 14 (2024): 276-286.
- Okutan E, Güleç I, Sengelen A, et al. Aloperine treatment attenuates acute spinal cord injury by reducing oxidative, inflammatory, and apoptotic responses via PI3K/AKT/NF-κB signaling in a rat contusion model. Neurosci Lett 854 (2025): 138203.
- Yao CH, Zhang ZM, Zhang S, et al. Berbamine alleviates neuropathic pain via suppressing spinal TMEM34/SGK1/FOXO3 axis. Phytomedicine 140 (2025): 156619.
- Li X, Yu H, Liu R, et al. Activation of the Nrf2 Signaling Pathway by Tetrahydroberberine Suppresses Ferroptosis and Enhances Functional Recovery Following Spinal Cord Injury. Mol Neurobiol (2025).
- Wang X, Huang W, Sun H, et al. Tomatidine relieves neuronal damage in spinal cord injury by inhibiting the inflammatory responses and apoptosis through blocking the NF-κB/CXCL10 pathway activation. Front Pharmacol 15 (2024): 1503925.
- Xu C, Jiang X, Yin W, et al. Microgel-encapsulated tetrandrine nanoparticles promote spinal cord repair by sustaining neuroinflammation inhibition. J Mater Chem B 13 (2025): 683-694.
- Tao J, Zhou J, Zhu H, et al. Tetramethylpyrazine inhibits ferroptosis in spinal cord injury by regulating iron metabolism through the NRF2/ARE pathway. Front Pharmacol 15 (2024): 1503064.
- Ma H, Xing C, Wei H, et al. Berberine attenuates neuronal ferroptosis via the AMPK-NRF2-HO-1-signaling pathway in spinal cord-injured rats. Int Immunopharmacol 142 (2024): 113227.
- Zhao Y, Ye C, Wang H, et al. Loading tea polyphenols enhances the repair of human umbilical cord mesenchymal stem cell sheet after spinal cord injury. Stem Cell Res Ther 16 (2025): 264.
- Alissa M, Alghamdi A, Alshehri A. Fibrin scaffold encapsulated with epigallocatechin gallate microspheres promote neural regeneration and motor function recovery after traumatic spinal cord injury in rats. Tissue Cell 93 (2025): 102691.
- Bagó-Mas A, Korimová A, Bretová K, et al. Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice. Int J Mol Sci 26 (2025): 3325.
- Li D, Dai Y, Li Z, et al. Resveratrol Upregulates miR-124-3p Expression to Target DAPK1, Regulating the NLRP3/Caspase-1/GSDMD Pathway to Inhibit Pyroptosis and Alleviate Spinal Cord Injury. J Cell Mol Med 29 (2025): e70338.
- Chen H, Zhao H. Resveratrol Enhances the Efficacy of Combined BM-MSCs Therapy for Rat Spinal Cord Injury via Modulation of the Sirt-1/NF-κB Signaling Pathway. Neurochem Res 50 (2024): 12.
- Zhong Q. Resveratrol enhances the protective effects of calcium supplements on spinal cord injury-induced osteoporosis by targeting the SIRT1/FOXO3a pathway. Naunyn Schmiedebergs Arch Pharmacol 398 (2025): 2823-2832.
- Bagó-Mas A, Korimová A, Deulofeu M, et al. Polyphenolic grape stalk and coffee extracts attenuate spinal cord injury-induced neuropathic pain development in ICR-CD1 female mice. Sci Rep 12 (2022): 14980.
- Mao J, Chen L, Zhuang G, et al. Curcumin-loaded polydopamine nanoparticles-based antioxidant scaffold promote spinal cord repair though dural-regulation of macrophage polarization. Mater Today Bio 32 (2025): 101895.
- Hadadi M, Farazi MM, Mehrabani M, et al. Curcumin reduces pain after spinal cord injury in rats by decreasing oxidative stress and increasing GABAA receptor and GAD65 levels. Sci Rep 15 (2025): 12910.
- He Y, Lu J, Luo Y, et al. Exploring the therapeutic mechanism of curcumin in spinal cord injury treatment based on network pharmacology, molecular dynamics simulation, and experimental validation. Front Chem 13 (2025): 1568551.
- Brazdzionis J, Radwan MM, Thankam FG, et al. A Swine Model of Changes in the Neuronal Electromagnetic Field After Traumatic Brain Injury: A Pilot Study. Cureus 15 (2023): e41763.
- Fu X, Zhang Y, Chen G, et al. Responsive nanoparticles synergize with Curcumin to break the “reactive oxygen Species-Neuroinflammation” vicious cycle, enhancing traumatic brain injury outcomes. J Nanobiotechnology 23 (2025): 172.
- Siahaan AMP. Ivander A, Ginting NRB, et al. Investigating the Impact of Turmeric on Neuroinflammation and Degenerative Changes in Repetitive Traumatic Brain Injuries: Insights from Murine Model. Korean J Neurotrauma 21 (2025): 18-31.
- Xu Y, Peng LS, Xiao CQ, et al. Bisdemethoxycurcumin mitigates traumatic brain injury in rats by modulating autophagy and oxidative stress via heat shock protein 90 alpha family class A member 1-mediated nuclear translocation of transcription factor EB. Brain Res Bull 222 (2025): 111221.
- Zhang J, Gu Y, Sun W, et al. Tetrahydrocurcumin Protects Against GSK3β/PTEN/PI3K/Akt-Mediated Neuroinflammatory Responses and Microglial Polarization Following Traumatic Brain Injury. Mol Neurobiol 61 (2024): 7026-7036.
- Li Z, Xu E, Zhang Y, et al. Deciphering spatiotemporal molecular pattern of traumatic brain injury by resveratrol-engineered two-dimensional-material-based field-effect-transistor biopatch. Biosens Bioelectron 279 (2025): 117360.
- Cao Q, Gu L, Wang L, et al. Resveratrol alleviates endoplasmic reticulum stress-induced cell death and improves functional prognosis after traumatic brain injury in mice. J Appl Biomed 22 (2024): 99-106.
- Asir F, Aslanoglu B, Tutal Gürsoy G, et al. Resveratrol showed anti-inflammatory effects on hippocampus via suppressing NFκB. Folia Morphol (Warsz) 83 (2024): 571-577.
- Zhou Z, Xiang W, Jiang Y, et al. Withaferin A alleviates traumatic brain injury induced secondary brain injury via suppressing apoptosis in endothelia cells and modulating activation in the microglia. Eur J Pharmacol 874 (2020): 172988.
- Zhu L, Li Z, Sheng L, et al. Ginkgolide A attenuated apoptosis via inhibition of oxidative stress in mice with traumatic brain injury. Heliyon 10 (2024): e24759.
- Liu X, Gu J, Wang C, et al. Ginsenoside Rg3 attenuates neuroinflammation and hippocampal neuronal damage after traumatic brain injury in mice by inactivating the NF-kB pathway via SIRT1 activation. Cell Cycle 23 (2024): 662-681.
- Tentu PM, Bazaz MR, Pasam T, et al. Oxyberberine an oxoderivative of berberine confers neuroprotective effects in controlled-cortical impact mouse model of traumatic brain injury. Int J Neurosci 135 (2025): 80-95.
- Cai Y, Zhang X, Qian H, et al. Uncovering the therapeutic efficacy and mechanisms of Quercetin on traumatic brain injury animals: a meta-analysis and network pharmacology analysis. Metabolic Brain Disease 40 (2024).
- Zhai X, Wang Z, Gao J. Quercetin alleviates microglial-induced inflammation after traumatic brain injury via the PGC-1α/Nrf2 pathway dependent on HDAC3 inhibition. Brain Res Bull 217 (2024): 111080.
- Cai Y, Zhang X, Zhang Q, et al. Ferulic Acid Alleviates Traumatic Brain Injury and Gastrointestinal Disorders by Promoting Ghrelin to Regulate the Microbiota-Brain-Gut Axis Inflammation and Pyroptosis. Phytother Res 39 (2025): 2291-2311.
- Zhang X, Cai Y, Chen M, et al. Danshen-Chuanxiong-Honghua ameliorates neurological function and inflammation in traumatic brain injury in rats via modulating Ghrelin/GHSR. J Ethnopharmacol 345 (2025): 119625.
- Ravikumar Reddy D, Khurana A, Bale S, et al. Natural flavonoids silymarin and quercetin improve the brain distribution of co-administered P-gp substrate drugs. Springerplus 5 (2016): 1618.
- Mitsunaga Y, Takanaga H, Matsuo H, et al. Effect of bioflavonoids on vincristine transport across blood–brain barrier1. European Journal of Pharmacology 395 (2000): 193-201.
- Liang G, Li N, Ma L, et al. Effect of quercetin on the transport of ritonavir to the central nervous system in vitro and in vivo, Acta Pharmaceutica 66 (2016): 97-107.
- He L, Zhao C, Yan M, et al. Inhibition of P-glycoprotein function by procyanidine on blood-brain barrier. Phytother Res 23 (2009): 933-937.
- Rejinold NS, Yoo J, Jon S, et al. Curcumin as a Novel Nanocarrier System for Doxorubicin Delivery to MDR Cancer Cells: In Vitro and In Vivo Evaluation, ACS Appl. Mater. Interfaces 10 (2018): 28458-28470.
- Reeta KH, Mehla J, Pahuja M, et al. Pharmacokinetic and pharmacodynamic interactions of valproate, phenytoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacology Biochemistry and Behavior 99 (2011): 399-407.
- Tang S, Wang A, Yan X, et al. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv 26 (2019): 700-707.
- Zhou J, Wu X, Zhao Z, et al. Huo, Preparation of a novel ginkgolide B niosomal composite drug. Open Chemistry 18 (2020): 1064-1074.
- Uchida S, Yamada H, Li XD, et al. Effects of Ginkgo Biloba Extract on Pharmacokinetics and Pharmacodynamics of Tolbutamide and Midazolam in Healthy Volunteers. The Journal of Clinical Pharmacology 46 (2006): 1290-1298.
- Chandra RH, Rajkumar M, Veeresham C. Pharmacokinetic Interaction of Ginkgo Biloba with Carbamazepine. Planta Medica 75 (2009): P-107.
- Johne A, Schmider J, Brockmöller J, et al. Roots, Decreased Plasma Levels of Amitriptyline and Its Metabolites on Comedication with an Extract From St. John’s Wort (Hypericum perforatum). Journal of Clinical Psychopharmacology 22 (2002): 46.
- Goey AKL, Meijerman I, Rosing H, et al. The Effect of St John’s Wort on the Pharmacokinetics of Docetaxel. Clin Pharmacokinet 53 (2014): 103-110.
- Garg SK, Kumar N, Bhargava VK, et al. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clinical Pharmacology & Therapeutics 64 (1998): 286-288.
- Hao M, Zhao Y, Chen P, et al. Structure-Activity Relationship and Substrate-Dependent Phenomena in Effects of Ginsenosides on Activities of Drug-Metabolizing P450 Enzymes. PLOS ONE 3 (2008): e2697.
- Zhou Y, Meng D, Chen F, et al. Inhibitory Effect of Imperatorin on the Pharmacokinetics of Diazepam In Vitro and In Vivo. Front Pharmacol 11 (2020): 01079.
- Bedada W, de Andrés F, Engidawork E, et al. The Psychostimulant Khat (Catha edulis) Inhibits CYP2D6 Enzyme Activity in Humans. J Clin Psychopharmacol 35 (2015): 694-699.
- Gupta YK, Gupta M, Chaudhary G, et al. Modulation of antiepileptic effect of phenytoin and carbamazepineby melatonin in mice. Methods and Findings in Experimental and Clinical Pharmacology 26 (2004): 99.
- Forcelli PA, Soper C, Duckles A, K. et al. Kondratyev, Melatonin potentiates the anticonvulsant action of phenobarbital in neonatal rats. Epilepsy Research 107 (2013): 217-223.
- Chroscinska-Krawczyk M, Jargiello-Baszak M, Walek M, et al. Caffeine and the anticonvulsant potency of antiepileptic drugs: experimental and clinical data. Pharmacological Reports 63 (2011): 12-18.
- Zanini C, Giribaldi G, Mandili G, et al. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and Ewing’s sarcoma cell lines. J Neurochem 103 (2007): 1344-1354.
- Jakubowicz-Gil E. Langner I, Wertel T. et al. Temozolomide, quercetin and cell death in the MOGGCCM astrocytoma cell line. Chemico-Biological Interactions 188 (2010): 190-203.

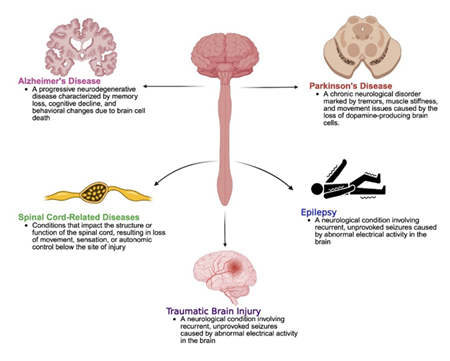
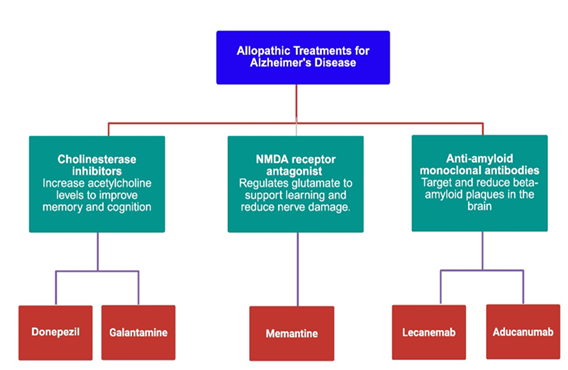
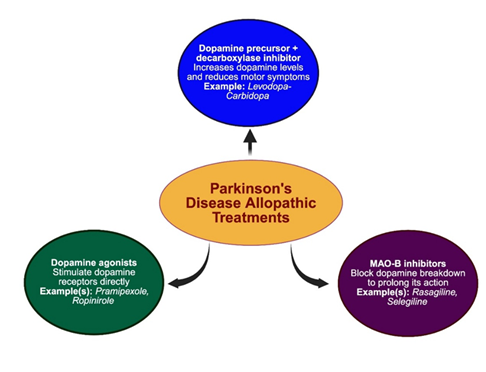
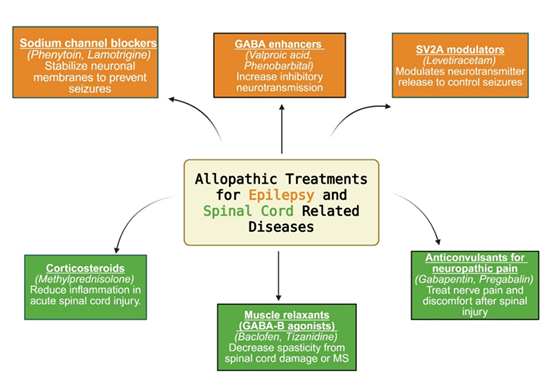
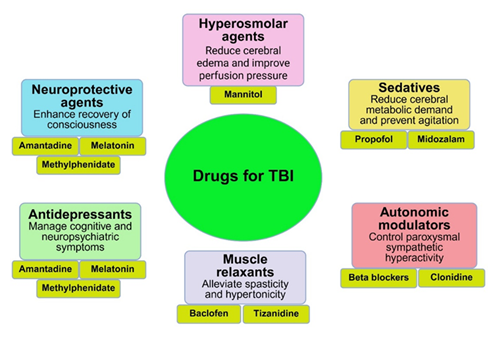
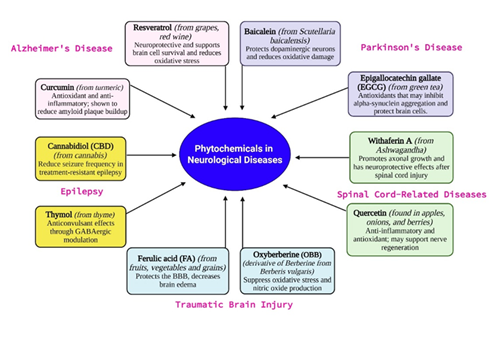

 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 