Reconstructive Therapy of Peri-Implantitis and Without Guided Bone Regeneration- A 15 Years Comparative Study
Dr. Abdullah Al Mamun Khan*1, Dr. Nasrin Parvin Zahan2
1Department of Oral and Maxillofacial Surgery, City Dental College & General Hospital, Dhaka, Bangladesh
2Dental Surgeon, Department of Oral and Maxillofacial Surgery, Dental and Implant Center, Dhaka, Bangladesh
*Corresponding author: Dr. Abdullah Al Mamun Khan, Department of Oral and Maxillofacial Surgery, City Dental College & General Hospital, Dhaka, Bangladesh, E-mail: drmamun@icloud.com
Received: 23 June 2025; Accepted: 30 June 2025; Published: 15 July 2025
Article Information
Citation:
Dr. Abdullah Al Mamun Khan, Dr. Nasrin Parvin Zahan. Reconstructive Therapy of Peri-Implantitis and Without Guided Bone Regeneration- A 15 Years Comparative Study. Fortune Journal of Health Sciences. 8 (2025): 695-699.
View / Download Pdf Share at FacebookAbstract
Background: Peri-implantitis is a challenging complication that compromises the long-term success of dental implants. Guided Bone Regeneration (GBR) has emerged as a promising reconstructive therapy, though long-term comparative data remain limited. This study aimed to compare the clinical outcomes and long-term implant survival between reconstructive therapy with and without GBR in peri-implantitis cases over a 15-year period.
Methods: This randomized controlled trial was conducted from 2009 to 2024 at two centers in Dhaka, Bangladesh. A total of 132 patients (76 males, 56 females; aged 30–70 years) diagnosed with peri-implantitis were included. Patients were randomly assigned to receive either non-regenerative therapy (n = 81) or regenerative therapy with GBR (n = 51). Exclusion criteria included uncontrolled systemic disease, heavy smoking, pregnancy, prior head/neck radiation, and poor follow-up compliance. Clinical and radiographic outcomes were evaluated over a 12-month period, with implant survival assessed up to 15 years.
Results: Baseline characteristics were comparable between groups (p > 0.05). At 12 months, the GBR group showed significantly greater probing depth reduction (3.6 ± 0.9 mm vs. 2.1 ± 0.8 mm; p < 0.001) and bone gain (2.9 ± 0.7 mm vs. 0.8 ± 0.5 mm; p < 0.001). Implant survival at 15 years was higher in the GBR group (82.4%) compared to the non-GBR group (69.1%). Post-operative complications were minimal in both groups.
Conclusion: GBR-based reconstructive therapy provides superior clinical and radiographic outcomes and enhances long-term implant survival compared to non-regenerative approaches in the management of peri-implantitis.
Keywords
<p style="text-align:justify">Peri-implantitis, Guided Bone Regeneration, Implant survival, Reconstructive therapy, Long-term outcomes, Bone regeneration. Re-osseointegration, Randomized controlled trial, Laser therapy</p>
Article Details
Introduction
Dental implants have become a widely accepted and predictable treatment modality for the replacement of missing teeth, offering long-term functionality and esthetic outcomes [1]. However, complications such as peri-implant diseases have emerged as significant challenges to implant success. Among these, peri-implantitis is a progressive, plaque-induced inflammatory condition characterized by soft tissue inflammation and progressive loss of supporting bone around an osseointegrated implant [2]. If left untreated, peri-implantitis may lead to implant failure. The prevalence of peri-implantitis varies widely, with estimates ranging from 10% to 47% depending on diagnostic criteria, patient-related risk factors, and duration of function [3, 4]. Contributing factors include poor oral hygiene, history of periodontitis, smoking, systemic diseases such as diabetes, biomechanical overload, and inadequate maintenance care [5]. Treatment of peri-implantitis is often complex due to the difficulty in accessing contaminated implant surfaces and the compromised regenerative potential of surrounding bone [6]. Several treatment modalities have been proposed for managing peri-implantitis, including non-surgical debridement, antiseptic application, systemic or local antibiotics, and surgical approaches [7]. Surgical therapy, particularly reconstructive techniques, aims not only to arrest disease progression but also to regenerate lost bone and restore implant function [8]. One of the most promising strategies in this regard is Guided Bone Regeneration (GBR), which combines mechanical decontamination with regenerative materials such as autogenous bone, bone substitutes, membranes, and biological enhancers like Platelet-Rich Fibrin (PRF) [9]. While many studies have reported short-term and medium-term outcomes of GBR in peri-implantitis management, long-term comparative data are limited [7, 10]. The effectiveness of GBR versus non-regenerative treatment in terms of clinical parameters such as probing depth reduction, radiographic bone gain, bleeding on probing, implant mobility, and long-term survival rates remains an area of ongoing research [11]. Furthermore, the risk-benefit profile of GBR—including potential complications like membrane exposure or graft resorption—needs to be evaluated over extended follow-up periods [12]. This study was designed to address this gap by evaluating and comparing the clinical and radiographic outcomes of reconstructive therapy with and without Guided Bone Regeneration in peri-implantitis cases over a 15-year period. The primary objective was to assess the long-term implant survival and bone regeneration outcomes associated with each approach. Secondary objectives included evaluating changes in probing depth, bleeding on probing, implant mobility, and the incidence of post-operative complications. By providing robust, long-term data from a large patient cohort, this study aims to inform evidence-based decision-making for clinicians managing peri-implantitis and contribute to optimizing treatment protocols in implant dentistry.
Methodology & Materials
This randomized controlled trial was conducted from 2009 to 2024 at two centers in Dhaka, Bangladesh: Banasree Dental and Implant Center and German Dental and Implant Center. Patients who had received implant treatment either at these centers or at other national or international clinics and subsequently developed peri-implantitis were evaluated for inclusion. Only patients who attended follow-up visits for at least one year were included in the study. Initially, 171 patients were assessed, of whom 39 were excluded due to lack of follow-up. Ultimately, 132 patients were enrolled, comprising 76 males and 56 females, with ages ranging from 30 to 70 years. Patients were randomly allocated into two groups: 81 patients received treatment without guided bone regeneration (GBR), and 51 patients received treatment with GBR. Exclusion criteria included patients with uncontrolled systemic diseases (e.g., diabetes, immunosuppression), active smokers exceeding 10 cigarettes/day, pregnant or lactating women, history of radiation therapy in the head or neck region, and those unwilling or unable to comply with follow-up requirements. In the non-GBR group, the implant surfaces were decontaminated using Piezo scalers with plastic tips, followed by diode laser application and local antiseptics (chlorhexidine). Systemic antibiotics—amoxicillin and metronidazole—were prescribed, and patients were monitored through regular follow-ups. In the GBR group, a full-thickness flap was elevated, and implant surfaces were cleaned mechanically with Piezo scalers and chemically decontaminated using saline and chlorhexidine. Laser surface modification was then performed, followed by bone grafting with autogenous bone or alloplastic material, platelet-rich fibrin (PRF), and a resorbable or non-resorbable membrane. These patients also received regular follow-ups. All clinical and radiographic data were collected and analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics, independent t-tests, and chi-square tests were used to compare baseline and post-treatment outcomes between groups. A p-value of <0.05 was considered statistically significant.
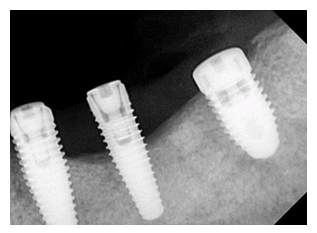
Figure 1: Clinical presentation of peri-implantitis at baseline showing inflammation, probing depth, and bone loss prior to intervention.
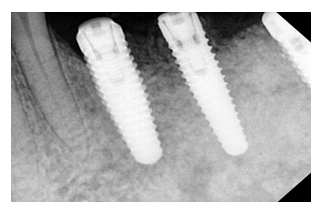
Figure 2: Clinical condition at 6-month follow-up demonstrating resolution of inflammation, reduced probing depth, and radiographic evidence of bone regeneration following GBR therapy.
Results
Table 1: Baseline Characteristics of Study Participants (n = 132)
|
Characteristics |
Without GBR (n = 81) |
With GBR (n = 51) |
p-value |
|
Age (years), mean ± SD |
52.3 ± 8.4 |
53.1 ± 7.9 |
0.48 |
|
Gender (Male/Female) |
47 / 34 |
29 / 22 |
0.96 |
|
Smoking status (Yes/No) |
21 / 60 |
13 / 38 |
0.88 |
|
Systemic disease (e.g., diabetes) |
12 (14.8%) |
8 (15.7%) |
0.91 |
|
Initial probing depth (mm) |
6.2 ± 1.1 |
6.5 ± 1.0 |
0.12 |
|
Initial bone loss (mm) |
4.8 ± 1.3 |
5.0 ± 1.4 |
0.39 |
Table 1 shows the baseline characteristics of the 132 patients, with 81 in the non-GBR group and 51 in the GBR group. Both groups were comparable in age, gender, smoking status, and presence of systemic disease (all p > 0.05). Initial clinical parameters, including probing depth (6.2 ± 1.1 mm vs. 6.5 ± 1.0 mm) and bone loss (4.8 ± 1.3 mm vs. 5.0 ± 1.4 mm), were also statistically similar between groups.
Table 2: Clinical Outcomes after 12 Months of Therapy
|
Outcome Measures |
Without GBR (n = 81) |
With GBR (n = 51) |
p-value |
|
Probing depth reduction (mm) |
2.1 ± 0.8 |
3.6 ± 0.9 |
<0.001 |
|
Bone fill (radiographic gain in mm) |
0.8 ± 0.5 |
2.9 ± 0.7 |
<0.001 |
|
Bleeding on probing (%) |
48.10% |
21.60% |
0.002 |
|
Suppuration (%) |
11.10% |
3.90% |
0.08 |
|
Implant mobility (%) |
9.80% |
2.00% |
0.04 |
|
Implant survival (%) |
90.10% |
98.00% |
0.03 |
Table 2 presents the clinical outcomes 12 months post-treatment. The GBR group showed significantly greater improvements in probing depth reduction (3.6 ± 0.9 mm vs. 2.1 ± 0.8 mm) and radiographic bone fill (2.9 ± 0.7 mm vs. 0.8 ± 0.5 mm), both with p < 0.001. Bleeding on probing was also notably lower in the GBR group (21.6% vs. 48.1%; p = 0.002). Although the reduction in suppuration did not reach statistical significance (p = 0.08), implant mobility was significantly less frequent in the GBR group (2.0% vs. 9.8%; p = 0.04), and implant survival was higher (98.0% vs. 90.1%; p = 0.03), indicating better overall treatment success with GBR.
Table 3: Long-Term Implant Survival (2009–2024)
|
Follow-Up Duration (Years) |
Without GBR Survival Rate (%) |
With GBR Survival Rate (%) |
|
1 Year |
90.10% |
98.00% |
|
5 Years |
85.20% |
94.10% |
|
10 Years |
78.30% |
88.20% |
|
15 Years |
69.10% |
82.40% |
Table 3 illustrates the long-term implant survival rates over a 15-year follow-up period. At each interval—1, 5, 10, and 15 years—the survival rate was consistently higher in the GBR group compared to the non-GBR group. At 15 years, implant survival was 82.4% in the GBR group versus 69.1% in the non-GBR group, indicating a substantial long-term benefit of guided bone regeneration in maintaining implant stability and longevity.
Table 4: Post-Operative Complications
|
Complication |
Without GBR (n = 81) |
With GBR (n = 51) |
p-value |
|
Post-op infection |
5 (6.2%) |
2 (3.9%) |
0.53 |
|
Membrane exposure |
N/A |
6 (11.8%) |
— |
|
Graft resorption |
N/A |
4 (7.8%) |
— |
|
Soft tissue recession |
9 (11.1%) |
4 (7.8%) |
0.49 |
Table 4 summarizes post-operative complications observed in both groups. The incidence of post-operative infection and soft tissue recession was slightly higher in the non-GBR group (6.2% and 11.1%, respectively) compared to the GBR group (3.9% and 7.8%), though differences were not statistically significant (p > 0.05). Unique to the GBR group were membrane exposure (11.8%) and graft resorption (7.8%), reflecting procedure-specific risks.
Discussion
This 15-year randomized controlled study compared the clinical and radiographic outcomes of reconstructive therapy for peri-implantitis with and without guided bone regeneration (GBR). Our findings demonstrate that GBR significantly improves clinical parameters, radiographic bone gain, and long-term implant survival compared to non-regenerative approaches. The GBR group showed a significantly greater mean probing depth reduction of 3.6 ± 0.9 mm, compared to 2.1 ± 0.8 mm in the non-GBR group (p < 0.001). Similarly, the radiographic bone fill was substantially higher in the GBR group (2.9 ± 0.7 mm) versus 0.8 ± 0.5 mm in the non-GBR group (p < 0.001). These findings align with those of Tomasi et al., who, in their meta-analysis, concluded that reconstructive therapy achieves superior defect fill and probing depth reduction in peri-implantitis cases [13]. Additionally, Renvert et al. also reported significantly better outcomes with reconstructive approaches at 3-year follow-up, reinforcing the durability of GBR-based interventions [14].
Implant survival was another key endpoint. At 15 years, implant survival in the GBR group was 82.4%, compared to 69.1% in the non-GBR group. This supports the long-term efficacy of regenerative techniques. Parma-Benfenati et al. similarly observed favorable implant survival in regenerative peri-implantitis therapy over 2–21 years, indicating that GBR may contribute to the preservation of implant function in the long term [15]. In terms of soft tissue health, bleeding on probing was significantly lower in the GBR group (21.6% vs. 48.1%, p = 0.002), consistent with Wang et al., who showed that laser-assisted regenerative therapy reduces inflammation and enhances clinical stability [16]. Although suppuration was lower in the GBR group (3.9% vs. 11.1%), it did not reach statistical significance (p = 0.08). However, the reduction in implant mobility (2.0% in GBR vs. 9.8% without GBR; p = 0.04) further supports improved peri-implant stability through regenerative therapy. The success of GBR is closely related to biomaterial selection and surgical technique. Our protocol involved the use of autogenous bone or alloplasts combined with platelet-rich fibrin (PRF) and membranes. Monje et al. emphasized the importance of combining barrier membranes with biomaterials for effective space maintenance and clot stabilization in defect regeneration [17]. Similarly, Castro et al. in their systematic review of RCTs highlighted that GBR consistently yields superior bone regeneration outcomes when biologically active materials such as PRF or enamel matrix derivatives are used [18]. While the GBR group demonstrated improved outcomes, procedure-specific complications were also noted. Membrane exposure occurred in 11.8% and graft resorption in 7.8% of GBR cases. These values are consistent with previous findings by Noelken and Al-Nawas, who identified membrane exposure as a frequent, though manageable, complication in GBR procedures [19]. Despite these occurrences, overall complication rates remained low and did not compromise implant survival or regenerative success. Interestingly, our study found that even non-regenerative treatments, involving mechanical debridement, diode laser, antiseptics, and antibiotics, resulted in moderate clinical improvement. This is in line with Ramanauskaite et al., who reported that surgical debridement alone can achieve short-term disease control, although long-term outcomes remain inferior to regenerative therapy [20]. A noteworthy finding from our study is the long-term benefit of GBR on implant survival. At 10 years, survival was 88.2% with GBR versus 78.3% without, and at 15 years, this gap widened further. This confirms the conclusions of Khoshkam et al., who found that regenerative therapies result in more favorable survival and radiographic outcomes over time [21]. The favorable results observed in our GBR group may also be influenced by the minimal invasiveness of the surgical technique. As discussed by Montero et al., minimally invasive regenerative approaches reduce soft tissue trauma and enhance healing outcomes, potentially explaining the lower rates of soft tissue recession in our GBR group (7.8% vs. 11.1%) [22].
Limitations of the study
While this study provides robust, long-term data, certain limitations must be acknowledged. The sample size disparity between groups, variability in implant systems and defect morphology, and the lack of histological analysis are factors that may influence generalizability. Nevertheless, the strength of the study lies in its 15-year follow-up, randomized design, and real-world clinical setting.
Conclusion
In conclusion, our findings support the use of guided bone regeneration as an effective and sustainable treatment modality for peri-implantitis. GBR significantly enhances clinical outcomes, promotes bone regeneration, and ensures greater long-term implant survival compared to non-regenerative approaches. These results are consistent with current literature and reinforce GBR as a key component in the surgical management of peri-implantitis.
Financial support and sponsorship
No funding sources.
Conflicts of interest
There are no conflicts of interest.
References
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions. Journal of clinical periodontology 45 (2018): S286-91.
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. Journal of clinical periodontology 42 (2015): S158-71.
- Renvert S, Persson GR, Pirih FQ, et al. Peri-implant health, Peri-implant mucositis, and Peri-implantitis: Case definitions and diagnostic considerations. Journal of clinical periodontology 45 (2018): S278-85.
- Mombelli A, Müller N, Cionca N. The epidemiology of Peri-implantitis. Clinical oral implants research 23 (2012): 67-76.
- Sanz M, Chapple IL, Working Group 4 of the VIII European Workshop on Periodontology*. Clinical research on Peri-implant diseases: consensus report of Working Group 4. Journal of clinical periodontology 39 (2012): 202-6.
- Philine Pfannenstiel H, Pandis N, Seehra J, et al. The Reporting Quality of Split-Mouth Studies in Implant Dentistry: A Survey. International Journal of Oral & Maxillofacial Implants 37 (2022).
- Santostasi N, Gerardi D, Rinaldi F, et al. Relationship between interleukin 1 (IL-1) genetic polymorphism and periimplantitis: systematic literature review and meta-analysis. European Review for Medical and Pharmacological Sciences 28 (2024): 3566-82.
- Lo Bianco L, Montevecchi M, Ostanello M, et al. Recognition and treatment of peri-implant mucositis: Do we have the right perception? A structured review. Dental and medical problems 58 (2021): 545-54.
- Monje A, Pons R, Roccuzzo A, et al. Reconstructive therapy for the management of Peri-implantitis via submerged guided bone regeneration: A prospective case series. Clinical implant dentistry and related research 22 (2020): 342-50.
- Ramanauskaite A, Becker K, Cafferata EA, et al. Clinical efficacy of guided bone regeneration in Peri-implantitis defects. A network meta-analysis. Periodontology 2000 93 (2023): 236-53.
- Donos N, Calciolari E, Ghuman M, et al. The efficacy of bone reconstructive therapies in the management of Peri-implantitis. A systematic review and meta-analysis. Journal of Clinical Periodontology 50 (2023): 285-316.
- Heitz-Mayfield LJ, Heitz F, Koong B, et al. Surgical Peri-implantitis treatment with and without guided bone regeneration. A randomized controlled trial. Clinical Oral Implants Research 34 (2023): 892-910.
- Tomasi C, Regidor E, Ortiz-Vigón A, et al. Efficacy of reconstructive surgical therapy at Peri-implantitis-related bone defects. A systematic review and meta-analysis. Journal of Clinical Periodontology 46 (2019): 340-56.
- Renvert S, Giovannoli JL, Rinke S. The efficacy of reconstructive therapy in the surgical management of Peri-implantitis: A 3-year follow-up of a randomized clinical trial. Journal of Clinical Periodontology 51 (2024): 1267-76.
- Parma-Benfenati S, Tinti C, Romano F, et al. Long-Term Outcome of Surgical Regenerative Treatment of Peri-implantitis: A 2-to 21-Year Retrospective Evaluation. International Journal of Periodontics & Restorative Dentistry 40 (2020).
- Wang CW, Ashnagar S, Gianfilippo RD, et al. Laser-assisted regenerative surgical therapy for Peri-implantitis: a randomized controlled clinical trial. Journal of periodontology 92 (2021): 378-88.
- Monje A, Pons R, Nart J, et al. Selecting biomaterials in the reconstructive therapy of Peri-implantitis. Periodontology 2000 94 (2024): 192-212.
- Castro F, Bouzidi AS, Fernandes JC, et al. Bone tissue regeneration in peri-implantitis: A systematic review of randomized clinical trials. The Saudi Dental Journal 35 (2023): 589-601.
- Noelken R, Al-Nawas B. Bone regeneration as treatment of Peri-implant disease: A narrative review. Clinical Implant Dentistry and Related Research 25 (2023): 696-709.
- Ramanauskaite A, Daugela P, de Almeida RF, et al. Surgical non-regenerative treatments for peri-implantitis: a systematic review. Journal of oral & maxillofacial research 7 (2016): e14.
- Khoshkam V, Chan HL, Lin GH, et al. Reconstructive procedures for treating peri-implantitis: a systematic review. Journal of dental research 92 (2013): 131S-8S.
- Montero E, Roccuzzo A, Molina A, et al. Minimal invasiveness in the reconstructive treatment of Peri-implantitis defects. Periodontology 2000 91 (2023): 199-216.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 